Abstract
Aims
This study assessed intraspecific variation in morphological traits of the fine root branch orders of Cryptomeria japonica, and identified variation in the diameter of the first three-order roots among species types with mycorrhiza, and the diameter of first-order roots at the family level.
Methods
Diameter, length, and specific root length of branch order roots (up to the fourth-order) were measured in intact fine root systems collected in four C. japonica stands. Relationships between soil chemical properties and morphological traits of the first- to fourth-order roots were investigated. The diameter of roots of 52 tree species reported in previous studies was compared at species types with mycorrhiza and at family level.
Results
The diameter of first-order roots in C. japonica varied by 1.2 times among stands. Negative correlations between soil NH4+ content and specific root length of the second- and third-order roots were observed in C. japonica. The diameter of first- and second-order roots forming arbuscular mycorrhiza in coniferous trees were significantly higher than those of roots forming ectomycorrhiza in coniferous and broadleaf trees. The diameter of first-order roots in Cupressaceae were significantly larger than those of Pinaceae, Sapindaceae, Betulaceae, and Fagaceae.
Conclusions
Clarifying intraspecific variation in morphological traits of C. japonica lower-order roots may contribute to understanding their responses to different site conditions such as soil inorganic nitrogen contents.
Similar content being viewed by others
Introduction
Fine roots, generally defined by a diameter of ≤2 mm and typically associated with mycorrhizal fungi, are responsible for nutrient uptake. They exhibit high physiological activity and play important roles in carbon (C) cycling in forest ecosystems (McCormack et al. 2015). Biomass, production, and turnover of tree fine roots have been investigated extensively in various biomes using diameter cut-offs to assess net primary productivity (NPP) in forest ecosystems (Finér et al. 2011a, 2011b; Brunner et al. 2013). Morphological traits of fine roots such as diameter, length, tissue density, or specific root length (SRL; length per dry weight unit) were shown to be sensitive to changing environments (Hirano et al. 2007; Ostonen et al. 2007; Kramer-Walter et al. 2016; Freschet and Roumet 2017). Nevertheless, results on fine root morphology are relatively scarce compared with studies focusing on biomass (McCormack et al. 2015), even though several recent studies assessed fine root traits from the viewpoint of the root economics spectrum (Kramer-Walter et al. 2016; Liese et al. 2017; Ma et al. 2018). The morphological traits of fine roots exhibit great variability depending on the branching position and are closely related to their functions, such as nutrient absorption and transport (Pregitzer et al. 2002; Guo et al. 2004, 2008; Wang et al. 2006; McCormack et al. 2015). However, previous studies on fine roots based on diameter cut-offs did not accurately describe their functions (McCormack et al. 2015), indicating that below-ground forest NPP estimations based on this classification may be insufficient to reflect the functions of roots.
Recently, a new classification of fine roots into two functional groups, absorptive and transport fine roots, has been proposed (McCormack et al. 2015). This classification is supported by anatomical differences in the ratio of cortical cell thickness to secondary growth of the stele (Tawa and Takeda 2015; Hishi et al. 2017). This classification overlaps with the branch order-based classification where the terminal roots are considered first-order roots and the second-order roots join two first-order roots, considering the branching structure (Pregitzer et al. 2002; Guo et al. 2004, 2008; Kong et al. 2014; Eissenstat et al. 2015; McCormack et al. 2015; Zadworny et al. 2017). The first two or three orders have been regarded as absorptive roots, as they possess intact cortical cells, whereas fine roots above the third or fourth order have secondary xylem and mainly function as transport elements (McCormack et al. 2015; Freschet and Roumet 2017).
Cryptomeria japonica and Chamaecyparis obtusa, two major plantation species covering up to 70% of the artificial forest area of Japan, are coniferous trees forming arbuscular mycorrhizas (AM) of Cupressaceae, and they are phylogenetically closely related. These two tree species have conventionally been planted on the same mountain slopes, with C. japonica typically on the moist lower slopes and C. obtusa on the drier middle to upper slopes. Thus, evaluation of the above- and below-ground ecological strategies for these species is of interest for forest management in Japan. Although extensive research has been conducted during the last decade on fine root morphology and particularly on root branch ordering of broadleaf species forming AM or ectomycorrhizas (EM) and coniferous species forming EM (Pregitzer et al. 2002; Guo et al. 2004, 2008; Chen et al. 2013; Kong et al. 2014; Eissenstat et al. 2015), only a few studies on this taxonomic type of AM-forming coniferous trees have addressed this topic (Tawa and Takeda 2015; Hishi et al. 2017; Doi et al. 2017).
Fine root traits regarding branch order have generally been assessed based on single stands (Pregitzer et al. 2002; Guo et al. 2004; Eissenstat et al. 2015), even though fine roots are considerably sensitive to stand and environmental conditions (Hirano et al. 2007, 2017; Miyatani et al. 2016; Doi et al. 2017). In fact, very few studies on intraspecific variations in fine root morphology in multiple stands of a given tree species have been conducted (Zadworny et al. 2016, 2017; Doi et al. 2017).
In C. obtusa, morphological traits of the fine root systems comprising lower (first three orders) and higher (fourth and higher) order roots up to 2 mm in diameter varied significantly among different three stands; in particular, roots of the first three orders were shown to be strongly correlated with soil inorganic nitrogen (N) content (Doi et al. 2017). Fine roots of C. japonica evaluated through diameter cut-offs also responded to the soil environment (Noguchi et al. 2013a, 2013b; Hirano et al. 2017). C. japonica specimens with lower specific root length (SRL) and higher biomass of fine roots of ≤0.5 mm in diameter were found in soils with low acid-buffering capacity, i.e., under low concentrations of exchangeable base cations and high aluminum (Al) content (Hirano et al. 2017). In contrast, Noguchi et al. (2013a) reported that the SRL of C. japonica roots of less than 1 mm in diameter increased with fertilization using high N doses (336 kg N ha−1 yr−1) over three years. The identification of C. japonica as a species that is particularly sensitive to its environment may help to clarify intraspecific variation in morphological traits associated with root branch order, and explain fluctuations observed in fine root turnover or the ratios of transport to absorptive fine roots induced by environmental and stand variables. Moreover, by clarifying the relationships between the ranges of root diameters determined by branch-order analysis and extensive data on fine root biomass based on diameter cut-offs (Konôpka et al. 2006; Fujimaki et al. 2007; Noguchi et al. 2007, 2013a, 2013b; Hirano et al. 2017), estimates of NPP in C. japonica stands may be improved.
The objectives of this study were to: (i) determine intraspecific variation in morphological traits of C. japonica fine roots as assessed using a branch order-based classification method, and (ii) investigate variation in the diameter of roots of the first three orders among species type with AM or EM, and the diameter of first-order roots at family level. To examine intraspecific variation, morphological traits were evaluated in four C. japonica stands by branch order analysis in intact fine root systems, and their relationships with surrounding soil chemical properties were tested. We discuss the contrast in the foraging strategies of lower order roots between two phylogenetically closely-related species, C. japonica and C. obtusa, grown at different soil inorganic N levels. The morphological traits of the first three orders of C. japonica were compared with those of 51 tree species as previously reported including coniferous and broadleaved trees forming AM or EM to elucidate variation among species types and evaluate the significance of diameter differences in first-order roots, particularly focusing on AM conifers and on families such as Cupressaceae from an evolutionary point of view and in light of a recent discourse by Ma et al. (2018).
Materials and methods
Study sites
This study was conducted in four Cryptomeria japonica stands located in the districts Chubu and Kansai in the central part of Japan: Hoki (34°56′ N, 135°36′ E), Kuroi (35°14′ N, 135°04′ E), Mayumi (34°18′ N, 136°20′ E), and Wakide (34°22′ N, 136°32′ E) (Table 1). The mean annual precipitation ranges from 1588 to 2221 mm, and the mean annual temperature was 13.5–15.6 °C. The surface geology of the Hoki, Kuroi, Mayumi, and Wakide stands was limestone, Paleozix, chert, and black schist, respectively. These stands were selected from among permanent monitoring plots established by the Forestry Agency of Japan in the 1990s (Forestry Agency of Japan 1997; Takahashi et al. 2001), based on the following criteria: tree species (C. japonica); soil type (Brown Forest Soils (Forest soil division 1976) and Cambisol (FAO-UNESCO 1990)); and comparable stand age and tree densities (Table 1). Each stand comprised a permanent plot of 0.1 ha (Forestry Agency of Japan 1997), and within the permanent a smaller plot of 10 m × 10 m was set up for intensive monitoring of changes in soil and fine roots (Tanikawa et al. 2014; Miyatani et al. 2016; Hirano et al. 2017).
Stand ages ranged from 54 to 69 years, and stand densities ranged from 700 to 1100 trees ha−1. Stem diameters at breast height ranged from 26 to 42 cm, and the total basal area ranged from 46 to 65 m2 ha−1. Only C. japonica is present in the canopy layer. The dominant understory species are Camellia sinensis at Hoki, Hydrangea luteovenosa and Thelypteris laxa at Kuroi, Edgeworthia chrysantha and Illicium anisatum at Mayumi and Machilus thunbergii at Wakide. The proportions of fine root biomass less than 2.0 mm in diameter of the understory to C. japonica in the mineral soil layer of 0–10 cm depth and in the organic layer were 17, 10, 3 and 1% in the Hoki, Kuroi, Mayumi and Wakide stands, respectively (Tanikawa et al., unpublished data; Table 1). The biomass of very fine roots less than 0.5 mm in diameter in C. japonica in the same soil layer ranged from 31.1 to 158.9 g m−2 (Tanikawa et al. unpublished data; Table 1).
Sampling of intact fine root systems and surface soil
Samples of intact fine root systems in the four stands of C. japonica were collected within one week (August 31 to September 6) in the summer of 2016. In each stand, five subplots of an area of 30 cm × 30 cm were established at 1-m distance from five selected C. japonica trees. The fine root system of C. japonica could be distinguished from the understory species by the differences in color, tips, and branching of roots. We followed the branch order classification of Pregitzer et al. (2002), which defines the distal roots as first-order roots, and roots at which two first-order roots intersect as second-order roots. Six intact fine root systems including fourth-order roots were harvested from the surface soils to a depth of 0–10 cm in each subplot.
A total of 30 intact fine root systems including fourth order were excavated carefully to avoid damaging root tips and were collected in each stand. Root systems were washed with tap water and distilled water to remove the adhering soil and organic matter and were wrapped in moistened paper at the field site to prevent drying. The samples were then placed in a cooling box and transported to the laboratory. Surface soil samples (down to 10 cm depth) including the organic layer were collected at the sampling points of the fine roots.
Analyses of intact fine root systems by the branch order classification
Intact fine root systems were cut at one point of the fourth-order root joined to the fifth-order root or even at larger-order roots; only intact fine root systems up to the fourth-order root were used for further analyses. Root systems were cut into individual roots at each intersection using tweezers and a scalpel, under a stereomicroscope (CP-745, AS ONE, Osaka, Japan). Images of all individual roots assigned to an order were acquired using a scanner (400 dpi, GT-X900, EPSON, Nagano, Japan). Average diameters and lengths of all individual roots within each root order in the intact root system were obtained by using the image analysis software WinRHIZO Pro, 2009c (Regent Instruments, Quebec, Canada). The number of all individual roots were counted manually on the scan. Subsequently, the individual roots were dried to constant weight at 60 °C for at least two days, and the dry weight in each root order in the intact root system was recorded.
From this data, specific root length (SRL) and root branching ratio, which are root morphological indicators, were calculated. SRL is root length per dry weight unit (expressed as m g−1) and represents the cost-benefit relationship of fine root production (Eissenstat and Yanai 1997). Higher SRL corresponds to thinner roots and a more frequent branching pattern, indicating efficient nutrient uptake (Ostonen et al. 2007). Root branching ratio (no. no.−1) was calculated as the number of the n-th order root joining to the (n + 1)th order root; this index represents the complexity of root branching (Wang et al. 2006). Schematic representations of fine root systems in the four C. japonica stands were reconstructed to exhibit and compare root architectures that was defined based on the above-mentioned data (average number, diameter, length, and branching ratio) of each root order, according to the method of Doi et al. (2017). The reconstructed root systems are assumed that the n-th order roots are only connected to the (n + 1)th order roots, even though some of the n-th order roots could be joined to the (n + 2)th, (n + 3)th or other branch orders in the field.
Soil chemical analysis
Air-dried soil samples were sifted using a 2-mm sieve to remove organic matter and gravel. Soil pH (H2O) and electrical conductivity (EC) were subsequently measured (soil:water, 1:2.5). After extraction with 50 mL 1 M KCl using 20 g of sieved soils, exchangeable Al concentration was determined by inductively coupled plasma atomic emission spectrometry (ICP-AES, SPS1500VR, Seiko, Chiba, Japan). Ten-gram aliquots of sieved soils were added to 100 mL of 2 M KCl, permeated for 1 h, and filtered. Soil NH4+ and NO3− were measured using indophenol (Ohyama 1990) and UV-absorption methods (Sakata 2000), respectively. Sieved soils were further pulverized finely using an agate mortar, and total soil C and N were measured using an NC analyzer (Sumigraph NC800, Sumika, Osaka, Japan). All concentrations were calculated on a dry-weight basis; soil samples were oven-dried at 105 °C for 24 h.
Diameter of lower order roots in different tree species
Data on diameters of lower-order (first to third) roots were compiled from previously published studies (Pregitzer et al. 2002; Wang et al. 2006; Guo et al. 2008; Jia et al. 2013; Eissenstat et al. 2015; Kubisch et al. 2015; Liu et al. 2015; Zadworny et al. 2015; Doi et al. 2017) to assess the extent to which the diameters of C. japonica lower-order roots differed from those of other tree species. Numerical values obtained from published papers were used; if diameters were shown only graphically (as part of figures), the image analysis software Digital Curve Tracer (1.0.0) (http://www.vector.co.jp/soft/win95/business/se174822.html) was used to produce numerical data. If multiple values per tree species were obtained, the data were averaged to produce a single value per species. Trees were divided into four species types using the criteria leaf type (broadleaf or coniferous) and mycorrhization pattern (arbuscular mycorrhiza, AM; or ectomycorrhiza, EM). The average values in each type were then calculated. We also compared the root diameters of C. japonica (assessed in the present study) with those of C. obtusa (Doi et al. 2017) because both are important species in Japanese coniferous plantations and are associated with AM. For families for which data were available for more than three species, the diameter of first-order roots at family level was also compared to consider an evolutionary point of view (Kong et al. 2014; Ma et al. 2018).
Statistical analyses
The average values of six root systems were calculated for each subplot. Based on five subplots, average values and standard errors of each stand were determined. The effects of four stands on fine roots traits by branch order, or on soil properties were analyzed using a one-way ANOVA; average values were compared among the four stands using a Tukey-Kramer test (n = 5). Pearson’s correlation coefficients (r) were calculated to analyze the relationship between each morphological trait (root diameter, root length, dry weight per individual root, SRL, and root branching ratio) and soil chemical content (NO3−, NH4+, N, and C) (n = 20, 5 subplots × 4 stands). The average diameters of the first three root orders of C. japonica were calculated and compared with those of C. obtusa as reported by Doi et al. (2017). The average diameters among the four types of trees (AM coniferous, AM broadleaf, EM coniferous, and EM broadleaf) were calculated for 52 species for which data was available. The average diameter of the first-order roots was calculated at the family level with more than three species per family, producing eight families with 39 tree species of the 52 species. Comparisons between C. japonica and C. obtusa, among the four types and among the eight families were performed using the Tukey-Kramer test. All data on root traits were transformed using the Box-Cox method to reduce heteroscedasticity after checking for normality using a Shapiro-Wilk test. All statistical analyses were performed using the software JMP Pro 13.0.0 (SAS Institute, USA).
Results
Intraspecific variation in morphological traits of root branch orders in C. japonica
A total of 10,763 individual roots of all 120 intact root systems (6 root systems × 5 subplots × 4 stands) in the four stands were examined. The average numbers of first-, second-, and third-order roots in intact fine root systems of C. japonica trees were (average ± S.E., n = 20; 5 subplots × 4 stands) 68.8 ± 2.9, 16.4 ± 0.7, and 3.5 ± 0.1, respectively. The average diameters of the first-, second-, third-, and fourth-order roots were 0.42 ± 0.01, 0.49 ± 0.01, 0.54 ± 0.01, and 0.69 ± 0.02 mm, respectively (Table 2); thus, roots belonging to the first two orders had a diameter of less than or about 0.5 mm. Diameters of the first- and second-order roots in the Hoki stand were significantly smaller than those in the Wakide stand (P < 0.05, n = 5).
The average length of individual roots tended to increase with increasing branch orders (Table 2), and there were no significant differences in this trait among the stands (P > 0.05, n = 5). First-order roots in the Hoki stand showed significantly higher SRL compared with first-order roots in the Wakide stand. Similarly, in the Hoki stand, SRL of second-order roots was significantly higher than those in the other three stands, whereas SRL of third-order roots significantly differed from that in the Mayumi and Wakide stands (Table 2, P < 0.05, n = 5). No significant differences in root branching ratio were observed among stands (Table 2, P > 0.05, n = 5).
The reconstructed fine root systems up to the fourth-order root revealed differences in C. japonica root architecture among the four stands (Fig. 1). The fine root system in the Hoki stand was the smallest whereas that in the Wakide stand was the largest regarding the contrast in diameter and SRL of the first three root branch orders (Table 2).
Schematic figures representing intact fine root systems (up to the fourth-order roots) of Cryptomeria japonica in four different stands, reconstructed from average diameters, lengths, and branching ratios. For the reconstruction of the root systems, it was assumed that the n-th order roots are only connected to the (n + 1)th order roots, even though some of the n-th order roots could be joined to the (n + 2)th, (n + 3)th or other branch orders in the field
Soil chemistry and effects on morphological root traits in C. japonica
Average values of soil pH (H2O) ranged from 3.9 to 4.2, and EC varied from 20.4 to 34.4 mS m−1; however, no significant differences were observed among the four stands (Table 3, P > 0.05, n = 5). The concentration of exchangeable Al in the Wakide stand was significantly higher than those in the Kuroi and Mayumi stands (P < 0.05). The concentration of soil NO3− in the Mayumi stand was significantly higher than those in the other stands, whereas soil NH4+ and C contents in the Wakide stand significantly exceeded those found in the Hoki stand (P < 0.05). No significant differences in soil N were observed among the four stands (P > 0.05).
The diameter of the first-order roots was positively correlated with total N and C concentrations in the soils when considering all subplots in the fours stands (Table 4, P < 0.05, n = 20; 5 subplots × 4 stands). In addition, significant positive correlations between the diameter of the second- or third-order roots and NH4+, total N or C concentrations in soils were found (P < 0.05). However, no significant correlations regarding root lengths (P > 0.05) were observed, apart from those found between the fourth-order roots and NH4+ and total C in soils (P < 0.05). There were negative correlations between the SRL of the second- or third-order roots and the concentrations of NH4+, total N or C in soils (P < 0.05).
Variation in the diameter of first-order roots among species type or family levels
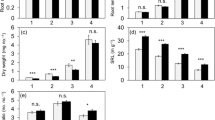
The diameters of the first- and second-order roots in AM-forming coniferous trees including C. japonica and C. obtusa were significantly higher than in EM-forming coniferous and broadleaf trees (Fig. 2, P < 0.05). The diameter of third-order roots of AM-forming coniferous trees was significantly larger than that of EM-forming broadleaf trees (P < 0.05). The diameters of the first- and second-order roots of C. japonica were significantly larger than those of C. obtusa (Supplementary Fig. 1, P < 0.05).
Average diameters of first- to third-order roots in broadleaved- or coniferous tree species with AM or EM (Pregitzer et al. 2002; Wang et al. 2006; Guo et al. 2008; Jia et al. 2013; Eissenstat et al. 2015; Kubisch et al. 2015; Liu et al. 2015; Zadworny et al. 2015; Doi et al. 2017; this study). (AM broadleaf trees, n = 28; EM broadleaf trees, n = 10; AM coniferous trees, n = 5; EM coniferous trees, n = 9). Different letters indicate significant differences in diameter in each root order among the four types (P < 0.05)
When the diameters of first-order roots were compared at family level (from families including at least three species among 52 tree species), the Cupressaceae family comprising four species including C. japonica and C. obtusa showed the second-largest diameter out of eight families and 39 tree species (Fig. 3). The average diameter in Cupressaceae exhibited no significant differences from that of Magnoliaceae, Lauraceae or Oleaceae (Fig. 3, P > 0.05), but was significantly larger than those of Pinaceae, Sapindaceae, Betulaceae and Fagaceae (Fig. 3, P < 0.05). In Cupressaceae, the first-order roots of Cunninghamia lanceolata had the largest diameter, followed by C. japonica, Juniperus monosperma, and C. obtusa (Supplementary Fig. 2).
Variation in the diameter of first-order roots in eight families. The data used were collected from the current study and the following published papers: Pregitzer et al. 2002; Wang et al. 2006; Guo et al. 2008; Jia et al. 2013; Eissenstat et al. 2015; Kubisch et al. 2015; Liu et al. 2015; Zadworny et al. 2015; Doi et al. 2017. Average diameter of first-order roots in eight families from 39 tree species among 52 tree species in Fig. 2 are shown because the data of other families were from only one or two tree species. Different letters indicate significant differences among families (P < 0.05)
Discussion
Intraspecific variation in lower-order roots of C. japonica
The proportion of absorptive to transportive fine roots affects the estimates of fine roots contribution to NPP because of their different turnover rates (McCormack et al. 2015). The results obtained in our study, which focused on the intraspecific variation in diameter of the first- and second-order roots, may be useful for estimating the biomass proportion in relation to absorptive roots, which have been found to have a shorter lifespan than transportive roots (McCormack et al. 2015), using the data on root biomass as categorized by diameter cut-offs. This study revealed that the diameters of the first- and second-order roots in the four C. japonica stands assessed were less than or about 0.5 mm. Primary roots with absorptive capacity for water and nutrients were anatomically observed only among the first- and second-order roots of C. japonica (Tawa and Takeda 2015; Hishi et al. 2017). The proportion of biomass of fine roots <0.5 mm in diameter to those <2.0 mm in diameter ranged from 10.7 to 50.3% in seven C. japonica stands in a previously published study (Hirano et al. 2017), and from 43.7 to 66.0% in the four stands in this study (Table 1, Tanikawa et al. unpublished data). Thus, these ranges would represent a similar proportion of absorptive to transportive fine roots in C. japonica.
Apart from its relevance for NPP estimates, the variation in diameter and biomass of absorptive roots is also important to understand how trees respond to different stand conditions and environmental factors. Although several studies focused on diameter variability of lower- (first- to third-) order roots among different tree species (Pregitzer et al. 2002; Guo et al. 2004; Eissenstat et al. 2015), there are very few studies on intraspecific variation among several stands, apart from some reports on Pinus sylvestris (Zadworny et al. 2016, 2017) and C. obtusa (Doi et al. 2017). In the present study, the diameter and SRL of all branch order roots in C. japonica among the four stands varied from 1.1- to 1.2- and from 1.2- to 1.7-times, respectively. Doi et al. (2017) investigated intraspecific variation in the first-order roots of C. obtusa among three stands and found that diameter and the SRL varied by 1.6- and 3.2-times, respectively. These results suggest that the intraspecific variation in diameter and SRL of the first-order roots in C. japonica occurs within a relatively small range. The diameter of first- and second-order roots in C. japonica were thicker than in C. obtusa in this study. Even though AM fungi exhibit only minor effects on root architecture compared with EM fungi (Maherali 2014), the colonization rate of AM in thicker lower-order roots could be higher than in thinner roots in temperate trees (Eissenstat et al. 2015). In a 90-year-old C. japonica stand, lower SRL of root tips with higher AM colonization rates was found compared to those in younger stands (Hishi et al. 2017). These results suggest that AM colonization could be one of the main drivers of intraspecific variations in root diameter and SRL in C. japonica.
Despite the small range of variation in C. japonica fine root diameters, significant relationships between morphological traits of root branch orders and soil C, N, or NH4+ were observed. In the Hoki stand, with the lowest C and NH4+ soil concentrations, the diameter and length of branch order roots were the smallest, but SRLs were the highest among the four stands sampled. Higher SRLs are related to thinner or more profusely branched roots (or both) and involve efficient nutrient uptake, counterbalancing the cost of root construction (Ostonen et al. 2007). SRL was orthogonal to the plant economic spectrum and thus, could increase or decrease infertile soils (Laughlin 2014; Kramer-Walter et al. 2016). Therefore, in C. japonica, thinner and smaller fine root systems in the Hoki stand may be adapted to lower, insufficient NH4+ or organic matter (C) content. Whereas higher SRLs require more C allocation because of a shorter lifespan and higher respiratory activity (Wang et al. 2013; Miyatani et al. 2018), roots could exhibit high SRL at any root tissue density (Laughlin 2014; Kramer-Walter et al. 2016). Under N-rich soil conditions, roots would not be needed to exhibit high SRL for N uptake (Jia et al. 2013). As fine root biomass in the Hoki stand was the least among the four stands, nutrient acquisition efficiency might be enhanced by increased SRL rather than by increased biomass.
The SRL of lower-order roots in C. japonica exhibited contrasting responses of those in C. obtusa to soil NH4+ (Fig. 4). Namely, negative correlations between soil NH4+ concentration and the SRLs of the second- and third-order roots were found in C. japonica, whereas positive correlations with SRL of the first-, second- and third-order roots were reported in C. obtusa (Doi et al. 2017, Fig. 4). These results suggest that SRL is independent from soil fertility gradients (Kramer-Walter et al. 2016) between different tree species in the present study. One explanation for this discrepancy may be linked to differences in nitrogen demand, despite C. japonica and C. obtusa being closely-related tree species of the Cupressaceae family. Plantations of C. japonica are conventionally found in nutrient rich soils but C. obtusa has been planted in soils with lower fertility (Tanikawa et al. 2014). Significant increases in transpiration rate, biomass, and N content in the leaves of C. japonica were found after seven-year treatments of N addition, but not in those of C. obtusa (Nagakura et al. 2008). These results suggest that C. japonica is more susceptible to soil N content than C. obtusa. Higher SRLs and thinner roots in C. japonica may be necessary to acquire nutrients more efficiently at lower nutrient soil availability. In contrast, new thinner root growth of C. obtusa might be promoted under soils with relatively higher inorganic N, leading to higher SRL. The results of this study suggest, therefore, that fine roots under nutrient limitations could exhibit different strategies even between closely-related tree species, such as C. japonica and C. obtusa. To elucidate the C investment strategy of absorptive roots for different nutrient availability, further studies are needed to clarify intraspecific variations not only in root traits but also in leaf and stem traits (Kramer-Walter and Laughlin 2017).
Correlation between SRL of second-order roots and soil NH4+ contents in Cryptomeria japonica and Chamaecyparis obtusa. The data of Chamaecyparis obtusa is from Doi et al. (2017)
It was reported that in the surface soil of a 28-year-old C. japonica stand, where NO3− increased as a consequence of continuous fertilization applications over three years, the SRL of roots of <1 mm in diameter increased and the biomass ratio of roots of <0.5 mm in diameter to roots of <1 mm in diameter was significantly higher than that in the control plot (Noguchi et al. 2013a). However, no relationships between soil NO3− and morphology of lower-order roots were found in this study. Although this contradictory finding remains to be explained, differences in soil chemical and physical properties between artificial experiments and natural conditions might be relevant, as differences in root growth strategies may occur within species under different soil environments. In addition, lower SRLs under higher soil N contents were observed in the first- to third-order roots of Pinus tabuliformis in artificial N-load experiments (Wang et al. 2013). The same trend was found in the present study regarding the relationship between SRLs of second- or third-order roots and NH4+ soil content. These findings indicate that there are different responses of SRL to soil N gradients (Kramer-Walter et al. 2016) even within one tree species such as C. japonica.
Our study also indicates that the second- and third-order roots were affected by the soil conditions, rather than the first-order roots, which are the most distal roots. This finding is consistent with results reported for lower-order roots of C. obtusa, where the SRL of second-order roots was more strongly correlated with soil inorganic N than the SRL of first-order roots (Doi et al. 2017). Chen et al. (2017) revealed that, in 5-year-old poplar plantations, the biomass of second-order roots, but not that of first-order roots, were significantly correlated with the content of soil available N or organic matters. One reason for this may be the longer exposure periods of second-order roots to the soil environment than that of first-order roots. An alternative explanation may be that the cortex of second-order roots is thicker, which is one of the root diameter-related traits and is related to mycorrhizal colonization rate (Kong et al. 2014), compared to first-order roots. In support of this observation is the fact that the intensity and frequency of AM colonization in second-order roots were higher than those in first-order roots in temperate trees (Eissenstat et al. 2015), suggesting that second-order roots may contribute to nutrient absorption capacity to a greater extent than most distal roots.
Variation in diameter of first-order roots among species type or family levels
We found that the diameter of first-order roots in Cupressaceae were in the range between those of Magnoliaceae and Lauraceae, which are AM broadleaf species (Fig. 3). Previous studies have shown that ancient angiosperms (such as Magnolia acuminata, Magnoliaceae) and gymnosperms (such as Taxus chinensis, Taxaceae) (Fig. 3, Supplementary Fig. 2) have relatively thick fine roots, which is a trait related to the evolutionary patterns of trees (Comas et al. 2012, 2014; Chen et al. 2013; Ma et al. 2018). Cupressaceae is phylogenetically closely related to Taxaceae (Kawahara 2014), and both conifer families have roots associated with AM fungi. This study clearly showed that the first-order roots of AM conifers have thicker diameters than those of EM conifers and broadleaf trees (Fig. 2). According to the study of Comas et al. (2014), in which 33 woody species associated with EM or AM (excluding AM-forming conifers) were examined, the diameters in roots associated with AM formation are thicker than EM-forming species, supporting the results obtained in this study (Figs. 2 and 3, Supplementary Fig. 2).
In the present study, we corroborated that in Cupressaceae, which form AM, the diameter of first-order roots decreased with evolution among three species (Cunninghamia lanceolata > C. japonica > C. obtusa), as proposed by Román-Jordán et al. (2017) (Supplementary Fig. 3), apart from J. monosperma which is a small shrubby tree species but was not considered regarding phylogenetic relationships (Brunsfeld et al. 1994; Gadek et al. 2000). This result on AM coniferous trees of the Cupressaceae family partly confirms the idea proposed by Ma et al. (2018) that thicker fine roots are coincident with evolutionary history, even within the family level.
However, it should be mentioned that only data on the diameter of first-order roots of five coniferous tree species with AM fungi (including C. japonica and C. obtusa, Cupressaceae) were collected in this study (Pregitzer et al. 2002; Liu et al. 2015; Doi et al. 2017), presumably due to the lack of more habitats of trees of this species type in Europe and North America. Therefore, further research is necessary to understand how lower-order root traits have changed as AM coniferous trees have evolved.
Conclusion
This study describes intraspecific variations in morphological traits of lower-order roots in four C. japonica stands. Furthermore, variation in the diameter of both first-order roots among family levels and the diameter of the first three-order roots among 52 tree species with AM or EM fungi is shown. Diameters of the first three-order roots in C. japonica were very similar to those reported for C. obtusa. Diameters of first- and second-order roots, as previously shown as absorptive roots (Tawa and Takeda 2015; Hishi et al. 2017), were less than or about 0.5 mm. Even closely-related species of coniferous trees of temperate zones forming AM such as C. japonica and C. obtusa exhibit different responses to soil inorganic N content, presumably owing to different nutrient requirements.
Clarifying how trees change their fine root traits to acquire soil nutrients is important to understand the responses of forest plantation trees to changing environments. Although we only examined morphological traits of lower-order roots in the current study, symbiotic relationships with mycorrhizal fungi are also relevant, as they regulate C investment and turnover in fine roots, and these relationships have not yet been investigated. Moreover, the relationships with stem and leaf traits (Kramer-Walter and Laughlin 2017) should be considered in future studies. The accuracy of NPP estimates for forest ecosystems could be enhanced by linking knowledge on fine root systems based on branch-order studies with accumulated biomass data based on diameter cut-offs (Doi et al. 2017).
Abbreviations
- Al:
-
Aluminum
- AM:
-
Arbuscular mycorrhiza
- C:
-
Carbon
- EC:
-
Electrical conductivity
- EM:
-
Ectomycorrhiza
- N:
-
Nitrogen
- NPP:
-
Net primary productivity
- SRL:
-
Specific root length
References
Brunner I, Bakker MR, Björk RG, Hirano Y, Lukac M, Aranda X, Børja I, Eldhuset TD, Helmisaari HS, Jourdan C, Konôpka B, López BC, Pérez CM, Persson H, Ostonen I (2013) Fine- root turnover rates of European forests revisited: an analysis of data from sequential coring and ingrowth cores. Plant Soil 362:357–372
Brunsfeld SJ, Soltis PA, Soltis DE, Gadek PA, Quinn CJ, Strenge DD, Ranker TA (1994) Phylogenetic relationships among the genera of Taxodiaceae and Cupressaceae: evidence from rbcL sequences. Syst Bot 19:253–262
Chen W, Zeng H, Eissenstat DM, Guo D (2013) Variation of first-order root traits across climatic gradients and evolutionary trends in geological time. Glob Ecol Biogeogr 22:846–856
Chen H, Dong Y, Xu T, Wang Y, Wang H, Duan B (2017) Root order-dependent seasonal dynamic in the carbon and nitrogen chemistry of poplar fine roots. New For 48:587–607
Comas LH, Mueller KE, Taylor LL, Midford PE, Callahan HS, Beerling D (2012) Evolutionary patterns and biogeochemical significance of angiosperm root traits. Int J Plant Sci 173:584–595
Comas LH, Callahan HS, Midford PE (2014) Patterns in root traits of woody species hosting arbuscular and ectomycorrhizas: implication for the evolution of below ground strategies. Ecol Evol 4:2979–2990
Doi R, Tanikawa T, Miyatani K, Hirano Y (2017) Intraspecific variation in morphological traits of root branch orders in Chamaecyparis obtusa. Plant Soil 416:503–513
Eissenstat DM, Yanai RD (1997) The ecology of root lifespan. Adv Ecol Res 27:2–60
Eissenstat DM, Kucharski JM, Zadworny M, Adams TS, Koide RT (2015) Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytol 208:114–124
FAO-UNESCO (1990) Soil map of the world revised legend. FAO, Rome
Finér L, Ohashi M, Noguchi K, Hirano Y (2011a) Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. For Ecol Manag 262:2008–2023
Finér L, Ohashi M, Noguchi K, Hirano Y (2011b) Factors causing variation in fine root biomass in forest ecosystems. For Ecol Manag 261:265–277
Forest Soil Division (1976) Classification of forest soils in Japan. Bull Gov For Exp Stn 280:1–28 (in Japanese with English summary
Forestry Agency of Japan (1997) Report for Forest damage-monitoring project by acid deposition (1990–1994), Tokyo (in Japanese)
Freschet GT, Roumet C (2017) Sampling roots to capture plant and soil functions. Funct Ecol 31:1506–1518
Fujimaki R, Tateno R, Tokuchi N (2007) Root development across a chronosequence in a Japanese cedar (Cryptomeria japonica D. Don) plantation. J For Res 12:96–102
Gadek PA, Alpers DL, Heslewood MM, Quinn CJ (2000) Relationships within Cupressaceae sensu lato: a combined morphological and molecular approach. Am J Bot 87:1044–1057
Guo DL, Mitchell RJ, Hendricks JJ (2004) Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 140:450–457
Guo D, Xia M, Wei X, Chang W, Liu Y, Wang Z (2008) Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol 180:673–683
Hirano Y, Mizoguchi T, Brunner I (2007) Root parameters of forest trees as sensitive indicators of acidifying pollutants: a review of research of Japanese forest trees. J For Res 12:134–142
Hirano Y, Tanikawa T, Makita N (2017) Biomass and morphology of fine roots in eight Cryptomeria japonica stands in soils with different acid-buffering capacities. For Ecol Manag 384:122–131
Hishi T, Tateno R, Fukushima K, Fujimaki R, Itoh M, Tokuchi N (2017) Changes in the anatomy, morphology and mycorrhizal infection of fine root systems of Cryptomeria japonica in relation to stand ageing. Tree Physiol 37:61–70
Jia S, Wang Z, Li X, Zhang X, Mclaughlin B (2013) Relationships between root respiration rate and root morphology, chemistry and anatomy in Larix gmelinii and Fraxinus mandshurica. Tree Physiol 33:579–589. https://doi.org/10.1093/treephys/tpt040
Kawahara T (2014) New group systems based on APG. For Genetic Tree Breed 3:15–22 (in Japanese)
Kong D, Ma C, Zhang Q, Li L, Chen X, Zeng H, Guo D (2014) Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol 203:863–872
Konôpka B, Noguchi K, Sakata T, Konôpková Z (2006) Fine root dynamics in a Japanese cedar (Cryptomeria japonica) plantation throughout the growing season. For Ecol Manag 225:278–286
Kramer-Walter KR, Laughlin DC (2017) Root nutrient concentration and biomass allocation are more plastic than morphological traits in response to nutrient limitation. Plant Soil 416:539–550
Kramer-Walter KR, Bellingham PJ, Millar TR, Smissen RD, Richardson SJ, Laughlin DC (2016) Root traits are multidimensional: specific root length is independent from root tissue density and the plant economic spectrum. J Ecol 104:1299–1310
Kubisch P, Hertel D, Leuschner C (2015) Do ectomycorrhizal and arbuscular mycorrhizal temperate tree species systematically differ in root order-related fine root morphology and biomass? Front Plant Sci 6:1–12
Laughlin DC (2014) The intrinsic dimensionality of plant traits and its relevance to community assembly. J Ecol 102:186–193
Liese R, Alings K, Meier IC (2017) Root branching is a leading root trait of the plant economic spectrum in temperate trees. Front Plant Sci 8:315. https://doi.org/10.3389/fpls.2017.00315
Liu B, Li H, Zhu B, Koide RT, Eissenstat DM, Guo D (2015) Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol 208:125–136
Ma Z, Guo D, Xu X, Lu M, Bardgett RD, Eissenstat DM, McCormack ML, Hedin LO (2018) Evolutionary history resolves global organization of root functional traits. Nature 555:94–97
Maherali H (2014) Is there an association between root architecture and mycorrhizal growth response? New Phytol 204:192–200
McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo D, Helmisaari H-S, Hobbie EA, Iversen CM, Jackson RB, Leppalammi-Kujansuu J, Norby RJ, Phillips RP, Pregitzer KS, Pritchard SG, Rewald B, Zadworny M (2015) Redefining fine roots improves understanding of belowground contributions to terrestrial biosphere processes. New Phytol 207:505–518
Miyatani K, Mizusawa Y, Okada K, Tanikawa T, Makita N, Hirano Y (2016) Fine root traits in Chamaecyparis obtusa forest soils with different acid buffering capacities. Trees 30:415–429
Miyatani K, Tanikawa T, Makita N, Hirano Y (2018) Relationships between specific root length and respiration rate of fine roots across stands and seasons in Chamaecyparis obtusa. Plant Soil 423:215–227
Nagakura J, Kaneko S, Takahashi M, Tange T (2008) Nitrogen promotes water consumption in seedlings of Cryptomeria japonica but not in Chamaecyparis obtusa. For Ecol Manag 255:2533–2541
Noguchi K, Konôpka B, Satomura T, Kaneko S, Takahashi M (2007) Biomass and production of fine roots in Japanese forests. J For Res 12:83–95
Noguchi K, Nagakura J, Kaneko S (2013a) Biomass and morphology of fine roots of sugi (Cryptomeria japonica) after 3 years of nitrogen fertilization. Front Plant Sci 4:1–7
Noguchi K, Nagakura J, Konôpka B, Sakata T, Kaneko S, Takahashi M (2013b) Fine-root dynamics in sugi (Cryptomeria japonica) under manipulated soil nitrogen conditions. Plant Soil 364:159–169
Ohyama T (1990) Inorganic nitrogen. In: editing committee of experimental methods for plant nutrition (ed) experimental methods for plant nutrition. Hakuyusha, Tokyo, pp 174–179 in Japanese
Ostonen I, Püttsepp Ü, Biel C, Alberton O, Bakker MR, Lõhmus K, Majdi H, Metcalfe D, Olsthoorn AFM, Pronk A, Vanguelova E, Weih M, Brunner I (2007) Specific root length as an indicator of environmental change. Plant Biosys 141:426–442
Pregitzer KS, Deforest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL (2002) Fine root architecture of nine north American trees. Ecol Monogr 72:293–309
Román-Jordán E, Esteban LG, de Palacios P, Fernández FG (2017) Comparative wood anatomy of the Cupressaceae and correspondence with phylogeny, with special reference to the monotypic taxa. Plant Syst Evol 303:203–219
Sakata T (2000) Quantification of NO3 −-N in soil extracts using UVabsorption method. Jpn J Environ 42:53–55 (in Japanese)
Takahashi M, Sakata T, Ishizuka K (2001) Chemical characteristics and acid buffering capacity of surface soils in Japanese forests Water Air Soil Pollut 130:727–732
Tanikawa T, Sobue A, Hirano Y (2014) Acidification processes in soils with different acid buffering capacity in Cryptomeria japonica and Chamaecyparis obtusa forests over two decades. For Ecol Manag 334:284–292
Tawa Y, Takeda H (2015) Which is the best indicator for distinguishing between fine roots with primary and secondary development in Cryptomeria japonica D. Don: diameter, branching order, or protoxylem groups? Plant Roots 9:79–84
Wang Z, Guo D, Wang X, Gu J, Mei L (2006) Fine root architec- ture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant Soil 288:155–171
Wang G, Fahey TJ, Xue S, Liu F (2013) Root morphology and architecture respond to N addition in Pinus tabuliformis, West China. Oecologia 171:583–590
Zadworny M, McCormack ML, Rawlik K, Jagodziński AM (2015) Seasonal variation in chemistry, but not morphology, in roots of Quercus robur growing in different soil types. Tree Physiol 35:644–652
Zadworny M, McCormack ML, Mucha J, Reich P, Oleksyn J (2016) Scots pine fine roots adjust along a 2000-km latitudinal climatic gradient. New Phytol 212:389–399
Zadworny M, McCormack ML, Żytkowiak R, Karolewski P, Mucha J, Oleksyn J (2017) Patterns of structural and defense investments in fine roots of scots pine (Pinus sylvestris L.) across a strong temperature and latitudinal gradient in Europe. Glob Chang Biol 23:1218–1231
Acknowledgements
We appreciate the constructive comments of the editor and three reviewers on the submitted manuscript. We also thank K. Noguchi (Forestry and Forest Product Research Institute, FFPRI) for reading the first draft of the manuscript and providing invaluable comments. We thank M. Takano, Y. Yamaguchi, T. Miyasaka (Nagoya University), T. Okamoto, T. Mizoguchi (FFPRI), and Y. Matsuda (Mie University) for their invaluable suggestions and assistance with fieldwork and laboratory analyses. We thank K. Yamase (Hyogo Prefectural Technology Center for Agriculture, Forestry and Fisheries), T. Kobayashi (Research Institute of Environment, Agriculture and Fisheries, Osaka Prefecture), and H. Fukumoto (The Mie Prefectural Forestry Research Center) for permission to access to the Forest Health-Monitoring Survey Sites of the Forestry Agency of Japan. This study was partly funded by JSPS KAKENHI Grant number 15H04519, 18 J23364 and 19H03011.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Boris Rewald.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 533 kb)
Rights and permissions
About this article
Cite this article
Wada, R., Tanikawa, T., Doi, R. et al. Variation in the morphology of fine roots in Cryptomeria japonica determined by branch order-based classification. Plant Soil 444, 139–151 (2019). https://doi.org/10.1007/s11104-019-04264-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04264-x