Post provided by Aelys Humphreys
Conifers for Christmas
It’s somehow fitting that the centre piece of an ancient midwinter tradition in Europe – that of decorating and worshipping an evergreen tree – is an ancient seed plant, a conifer. In Europe, we tend to think of conifers as “Christmas trees” – evergreen trees with needles and dry cones, restricted to cold and dry environments – but conifers are much more diverse and widespread than that. There are broad-leaved, tropical conifers with fleshy cones and even a parasitic species that is thought to parasitise on members of its own family!

However, while today’s distribution of conifers is global – spanning tropical, temperate and boreal zones – it is fragmented. The conifer fossil record extends well into the Carboniferous and bears witness to a lineage that was once much more abundant, widespread and diverse. So we can tell that today’s diversity and distribution have been shaped by hundreds of millions of years of speciation, extinction and migration.
Shared Evolutionary History and Fate in Conifers?
How have the events that make up conifer evolutionary history been patterned, phylogenetically and geographically? Have speciation and extinction occurred equally across all conifers? Or have certain lineages or geographical regions seen higher rates of species turnover? If so, are conifers in certain lineages or regions more prone to extinction in the future?
Being ancient and widespread, it’s unlikely that all conifer lineages have experienced the same diversity dynamics in the past, nor is it likely that they share the same fate. For example, the two species that are commonly used as Christmas trees, Norway spruce (Picea abies) and the Nordmann fir (Abies nordmanniana), belong to different genera but the same family (Pinaceae). They share a lot of their evolutionary history but not all. And what about fate? We tend not to think about shared fate for groups above the species level but could the shared family membership of Christmas trees mean a shared evolutionary fate? On a coarse level, species turnover has been found to be higher in Northern than Southern Hemisphere conifers. What does that mean for the individual lineages that occupy each hemisphere? And what processes can cause such variation in speciation and extinction probabilities across higher clades?
— Do the Christmas trees Norway spruce and Nordmann fir share the same evolutionary fate? (Images show immature female cones.)
Evolutionarily Significant Units of Diversity Above the Level of Species
Simulations have shown that some processes that lead to the formation of discrete species (e.g. geographical isolation and divergent selection) can lead to the formation of discrete higher clades as well (see also Barraclough 2010). This occurs if the number of species that can co-occur in a geographical region or ecological zone is limited; there is species turnover through ongoing speciation and extinction; and species migration between different regions or zones is rare. Under this scenario, species will fall into a set of discrete clades, which we call higher evolutionarily significant units (hESUs), each occupying a separate region or zone. Due to species turnover, members of a hESU share evolutionary history as well as evolutionary fate. Any event influencing the likelihood of speciation or extinction will be shared among species within an hESUs, but not among species in different hESUs. This means that speciation and extinction probabilities are shared within hESUs but decoupled among them.
Evidence for Higher Evolutionarily Significant Units of Diversity in Conifers
The phylogenetic signature of hESUs is a long stem branch leading to each discrete clade. This produces a significant upturn in a lineages-through-time (LTT) plot. We can see this pattern in the conifer phylogeny; but, diversification into hESUs is not the only process that can lead to such a pattern.

In our recent paper – Detecting evolutionarily significant units above the species level using the generalised mixed Yule coalescent method – we tested whether it is possible to rule out alternative explanations for the conifer LTT curve. To test our ability to detect hESUs we simulated trees under two models that can lead to similar phylogenetic patterns. While the hESU model is clade-dependent – it considers both diversification rates and the clades in which they occur – the alternative two models are clade-independent and consider diversification rates that are shared among all members of a wider clade. Rates may be constant over time or vary:
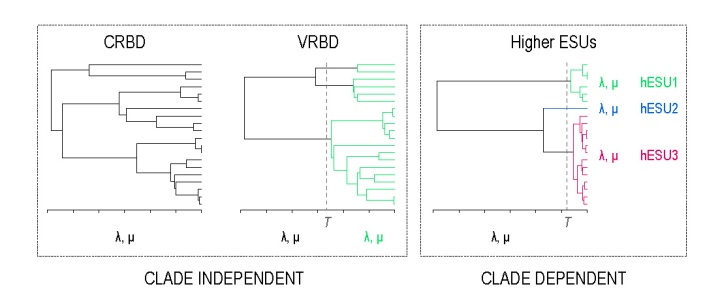
To detect hESUs we use the generalised mixed Yule coalescent method, originally developed for delimiting species. When we applied this method to trees simulated under the two clade-independent models, we found high error rates – i.e. high rates of erroneous support for the existence of hESUs – especially if trees were simulated with high rates of relative extinction. We were able to rule out the constant-rates birth-death model as an explanation for the patterns in conifers though because support for the hESU model was much higher on the conifer tree than on simulated trees. Unsurprisingly, it was not possible to rule out the variable-rates birth-death model – in which a clade-wide increase in rates occurs – in this way. Instead we compared the simulated trees to the conifer tree and found that they differed significantly in several tree metrics: simulated trees were older, more balanced and more stemmy.
We concluded that there is evidence that conifers have diversified into hESUs but that, in most cases, it may only be possible to rule out alternative models by using simulations and additional tree metrics. Future studies may incorporate additional metrics into the model-fitting framework.
Do Christmas Trees Share Evolutionary Fate?
The overwhelming majority of hESUs detected in conifers correspond to genera and date to the Miocene. Correspondence with named higher taxa is striking in other groups of gymnosperms and in mammals as well. Intriguingly, the average age of hESUs detected in widely disparate groups of organisms dates to the Miocene, suggesting similar rates of species turnover across clades and regions.
Returning to our Christmas trees, the Norway spruce and Nordmann fir, it would seem that their separate genus membership is indicative of them following their own evolutionary trajectories, at both species and higher levels. In mammals, hESUs correspond with variation in body mass and dentition, consistent with hESUs being established following radiation into novel adaptive zones. The two Christmas tree genera, Abies and Picea, have largelly overlapping ranges and similar gross morphology; so, at the moment it’s unclear what has caused diversification into hESUs in conifers.
So, what’s the take-home message to ponder while you decorate your tree? Taking into account diversification rates, as well as the clades in which they occur, can tell us more about the process of evolution. It provides a means for delimiting evolutionarily real higher units that share not only evolutionary history but evolutionary fate as well. This approach provides a crossroads between taxonomy and evolution, which is generally lacking above the level of species.
Happy Holidays, Everyone!
To find out more about how to study higher evolutionarily significant units, read our Methods in Ecology and Evolution article ‘Detecting evolutionarily significant units above the species level using the generalised mixed Yule coalescent method’.




Nice article, but the fir pic is Korean Fir Abies koreana, not A. nordmanniana 😉