当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
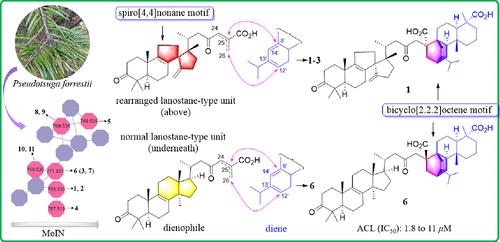
Forrestiacids E–K: Further [4 + 2]-Type Triterpene–Diterpene Hybrids as Potential ACL Inhibitors from the Vulnerable Conifer Pseudotsuga forrestii
Journal of Natural Products ( IF 5.1 ) Pub Date : 2023-05-17 , DOI: 10.1021/acs.jnatprod.3c00040 Peng-Jun Zhou 1, 2 , Ting Huang 2 , Guang-Lei Ma 2 , Ying-Peng Tong 1 , Wen-Xue Chen 3 , Yi Zang 4 , Juan Xiong 2 , Jia Li 4 , Jin-Feng Hu 1, 2
Journal of Natural Products ( IF 5.1 ) Pub Date : 2023-05-17 , DOI: 10.1021/acs.jnatprod.3c00040 Peng-Jun Zhou 1, 2 , Ting Huang 2 , Guang-Lei Ma 2 , Ying-Peng Tong 1 , Wen-Xue Chen 3 , Yi Zang 4 , Juan Xiong 2 , Jia Li 4 , Jin-Feng Hu 1, 2
Affiliation
|
Seven [4 + 2]-type triterpene–diterpene hybrids derived from a rearranged or a normal lanostane unit (dienophile) and an abietane moiety (diene), forrestiacids E–K (1–7, respectively), were further isolated and characterized from Pseudotsuga forrestii (a vulnerable conifer endemic to China). The intriguing molecules were revealed with the guidance of an LC-MS/MS-based molecular ion networking strategy combined with conventional phytochemical procedures. Their chemical structures with absolute configurations were established by spectroscopic data, chemical transformation, electronic circular dichroism calculations, and single-crystal X-ray diffraction analysis. They all contain a rare bicyclo[2.2.2]octene motif. Both forrestiacids J (6) and K (7) represent the first examples of this unique class of [4 + 2]-type hybrids that arose from a normal lanostane-type dienophile. Some isolates remarkably inhibited ATP-citrate lyase (ACL), with IC50 values ranging from 1.8 to 11 μM. Docking studies corroborated the findings by highlighting the interactions between the bioactive compounds and the ACL enzyme (binding affinities: −9.9 to −10.7 kcal/mol). The above findings reveal the important role of protecting plant species diversity in support of chemical diversity and potential sources of new therapeutics.
更新日期:2023-05-17



























 京公网安备 11010802027423号
京公网安备 11010802027423号