The number and size of the stomata
by Eckerson S. F. (1908)
in Bot. Gaz. 46: 221-224. –
https://archive.org/stream/jstor-2467475/2467475_djvu.txt
………………
P. 224:
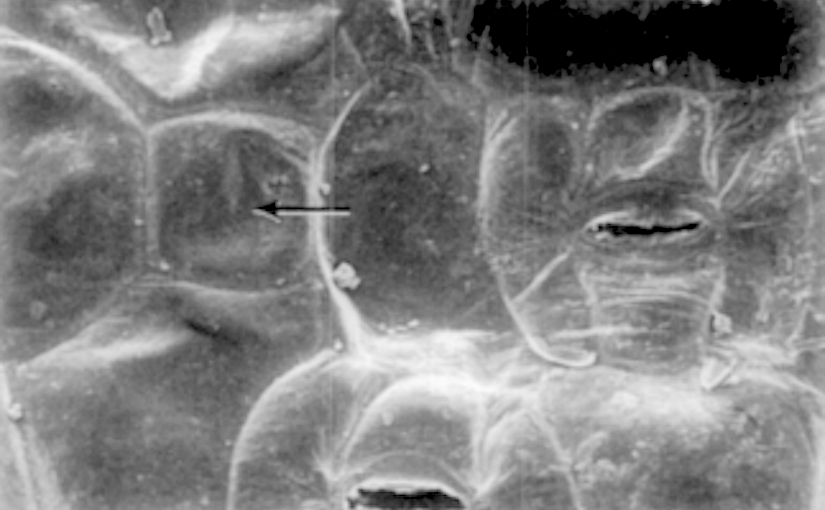
Counting or measuring the stomata in situ on the leaf is possible with a few plants, notably Begonia coccinea, Chrysanthemum frutescens, Fuchsia speciosa, Impatiens Sultani, Primula obconica, Pelargonium zonale, Trades- cantia zebrina, and Vicia Faba. In some others the condition of the pore can thus be observed, though the outlines of the guard cells are not clear: this is true in Senecio Petasitis, Helianthus annuus, Cyclamen latijolium, Coleus Blumei, Cestrum elegans, and Phaseolus vulgaris. Marked variations in number and size of stomata occur, not only in different varieties of the same species, but in the same varieties grown under different external conditions. So far as my observation goes, however, the variation is greater in number than in size. Furthermore, while in most leaves the stomata are fairly evenly distributed over the surfaces containing them, in some, especially in oblong leaves (e. g., Fuchsia speciosa, Helianthus annuus, and Impatiens Sultani), the stomata are much more numerous near the base than near the tip (more than twice as many), and near the midrib than near the margin. For this reason very different figures might be given for the same leaf by different observers. The opening and closing of the stomata of greenhouse plants is corre- lated closely with the time of day, and secondarily with the weather. As already noted, they are, as a rule, as wide open as they can be about 10 A. m. — this, of course, in well-watered plants. In favorable weather they remain wide open until about 2 .30 p. m., when they begin to close, and they are mostly completely closed by 5 p. m., though some may remain open until 6. On hot days in the spring they may close as early as 12 m., probably because of incipient wilting of the leaf. If the stomata are closed by wilting, they may be made to open, partially at least, by immersion of the leaf in water. The best plants for general laboratory study, taking account of ease of removing the epidermis, size and clearness of stomata, and commonness of occurrence in greenhouses, are, in order of excellence, Chrysanthemum frutescens, Tradescantia zebrina, Pelargonium zonale, Fuchsia speciosa, Helianthus annuus, and Vicia Faba. — Sophia H. Eckerson, Smith College, Northampton, Mass.










You must be logged in to post a comment.