First Fossil Fokienia (Cupressaceae) in South China and Its Palaeogeographic and Palaeoecological Implications
- 1State Key Laboratory of Biocontrol and Guangdong Provincial Key Laboratory of Plant Resources, School of Life Sciences/School of Ecology, Sun Yat-sen University, Guangzhou, China
- 2Geological Institute, Russian Academy of Sciences, Moscow, Russia
- 3Borissiak Paleontological Institute, Russian Academy of Sciences, Moscow, Russia
- 4School of Earth Science and Resources, Chang’an University, Xi’an, China
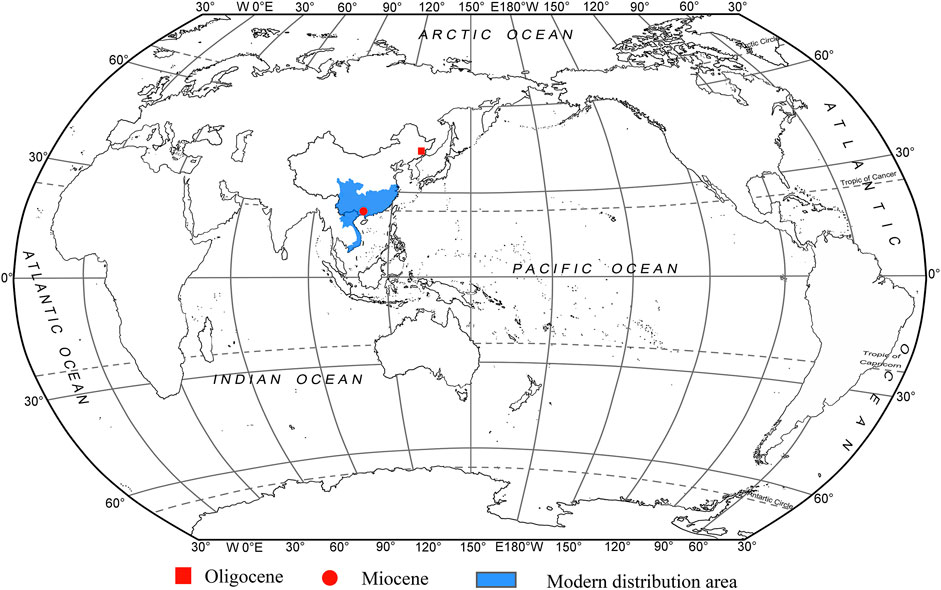
Fokienia A. Henry & H. H. Thomas is a monotypic genus of the Cupressoideae Rich. ex Sweet (Cupressaceae), native to subtropical evergreen mesophytic forests in South China, northern Laos and Vietnam. The fossil record of Fokienia is very scanty, with only one known occurrence of foliage in the Oligocene of Longjing, Jilin, China. Here we report the fossil foliage of Fokienia discovered in the Miocene Erzitang Formation of the Guiping Basin, South China, which is similar to that of the only extant species Fokienia hodginsii in both macromorphological and epidermal features. This species is the earliest fossil record within the modern distribution area of Fokienia and the only fossil species for which morphology and anatomy have been studied in detail. Fossil evidence suggests that the genus Fokienia was present at middle latitudes of the Northern Hemisphere in the Oligocene and spread to South China during the Miocene. Due to physiological adaptations to warm-wet environments and weak cold tolerance, Fokienia migrated southward, as global climate cooling gradually drove it to extinction in the mid-latitudes. The ecological niche of the extant species, and co-existing plant fossils, suggest that the fossil assemblage represents the remains of an evergreen broad-leaved and conifer mixed forest growing under humid and warm Miocene climate.
Introduction
The monotypic genus Fokienia A. Henry et H. H. Thomas belongs to the subfamily Cupressoideae Rich. ex Sweet within the Cupressaceae. The only extant species, Fokienia hodginsii (Dunn) Henry & Thomas is an evergreen tree species native to subtropical evergreen mesophytic forests in South China (except Hainan Island), northern Laos and Vietnam (Figure 1; Farjon, 2005). Here, Fokienia has significant economic value, its high-quality timber is extensively used for building materials, furniture construction and fine handicrafts. This conifer is also used in forest plantations, and its valuable essential oil is used in pharmaceuticals and cosmetics (Fu et al., 1999).
Fokienia hodginsii was firstly treated as a new species of Cupressus (sect. Chamaecyparis) hodginsii by Dunn (1908), and then Henry and Thomas (Henry, 1911) assigned this species to a new genus Fokienia based on the morphological characteristics of its seed cones, seeds and leaves. Recently extant species are sometimes referred as Chamaecyparis hodginsii (Dunn) Rushforth (Rushforth, 2007). Based on detailed analyses of the ontogeny of the seed cones (Farjon, 2005; Jagel and Dörken, 2015), morphological phylogenetic analysis (Farjon, 2005) and DNA sequence data (Gadek and Quinn, 1993), Fokienia is now considered to be separate from Chamaecyparis. Recent molecular phylogenetic analyses cluster these two genera into single subclade in Cupressaceae (Yang et al., 2012) and strongly support the sister relationships between Chamaecyparis p. p. and Fokienia (Gadek et al., 2000). However, combined analysis of molecular data and morphological characters revealed the varying placement of Fokienia hodginsii within the phylogenetic tree according to different sequence data (Little et al., 2004).
Fokienia is similar to most genera of the subfamily Cupressoideae in terms of leaf macromorphology, but can be effectively distinguished by the epidermal characteristics (Kvaček et al., 2000; Kvaček and Rember, 2007; Shi et al., 2011, 2012). Moreover, the macromorphology and anatomical characteristics of Cupressoideae foliage have been studied in detail (Kvaček et al., 2000; Wang, 2007; Shi et al., 2011, 2012; Wu et al., 2014, 2019; Zhang et al., 2015), which plays an important guiding role in the identification of Fokienia.
Fossils of Fokienia are very rare (Figure 1). Foliage of Fokienia sp. from the Oligocene Sanhe flora of Longjing, Jilin, China, is morphologically similar to that of extant F. hodginsii and seems to be the only reliable fossil record of this genus, but its leaf epidermal anatomy remains unknown. In this paper, we describe a new fossil species of Fokienia on the basis of foliage from the Miocene of South China and discuss the phytogeographical and palaeoecological implications of this genus.
Materials and Methods
The fossils studied here were collected from the Miocene Erzitang Formation in the Guiping Basin located east of Guiping City, Guangxi, South China. The fossil locality is on the northeastern edge of this basin, near Longsheng Village, Tianping Town, Tengxian County (Figure 2, 23°22′55.04″N, 110°44′13.31″E). The Erzitang Formation in this area consists of coal-bearing deposits. Fokienia leafy shoots and isolated leaves along with numerous other plant fossils are preserved as compressions in gray-black shales. Based on the mammal genus Prolipotes yujiangensis Zhou, Zhou & Zhao found in the Guiping Basin, Zhou et al. (1984) and Zhao (1988) suggested a Miocene age for the Erzitang Formation. Extant specimens were collected from Daiyunshan National Nature Reserve in Fujian Province, China and Bidoup-Nui Ba National Park of Hàn Giao forest station in Đà Lạt District, Lâm Đồng Province, Vietnam.
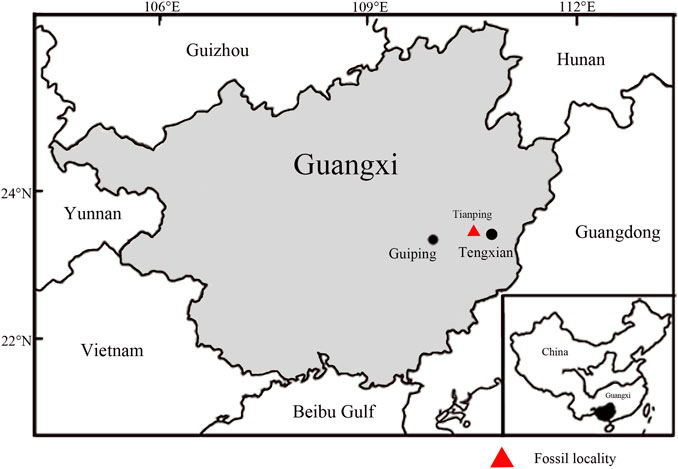
FIGURE 2. Map showing the fossil locality (red triangle, modified from Han et al., 2018).
Fossil and extant specimens were photographed using a Canon EOS 500D digital camera (Canon, Tokyo, Japan), and images were processed with DigiCamControl-Free Windows DSLR camera controlling solution (Duka, 2015) and Helicon Focus 6.6.1 (Helicon Soft Ltd., Kharkov, Ukraine). Fossils were treated by immersion in 10% hydrochloric acid for 12h, then in Schulze’s solution (70% HNO3, saturated with KClO3) for approximately 1 h followed by 5–10% KOH for 30 min (Ye, 1981). After each process the samples were washed with distilled water. The cuticles were separated with a needle under a stereomicroscope. The leaves of extant F. hodginsii were boiled to translucency in a mixed solution of 10% acetic acid and 10% H2O2 (1:1; Lin, 2007). After treatment with Schulze’s solution followed by 8% KOH for 30 min each, the cuticles were separated. The cuticles of fossil and modern leaves were stained with 1% fuchsin solution and mounted on glass slides in glycerine jelly for observation and photography using an Axio Scope A1 stereoscopic microscope (Carl Zeiss, Oberkochen, Baden-Wurttemberg, German). Parts of unstained fossil and extant cuticles were mounted on stubs, then observed and photographed using a JSM-6330F scanning electron microscope (JEOL Ltd., Akishima, Tokyo, Japan) with an accelerating voltage of 10 keV. All the specimens are deposited in the Museum of Biology, Sun Yat-sen University with collection numbers beginning with TX.
Systematic Palaeontology
Family Cupressaceae Gray, 1822
Subfamily Cupressoideae Rich. ex Sweet, 1826
Genus Fokienia A. Henry & H. H. Thomas, 1911
Fokienia tianpingensis Wu & Jin, sp. nov.
Figures 3A,C,E–G; Figure 4; Figures 5A–D.

FIGURE 3. Foliage of Fokienia tianpingensis sp. nov. (A, C, E–G) and extant species F. hodginsii (Dunn) Henry et Thomas (B, D, H). (A) Branchlets of two orders, showing the alternate branching pattern, holotype TX-2–1. (B) Similar ultimate branchlets of extant species. (C) Incompletely preserved shoot bearing penultimate branchlets, TX-38–1. (D) Similar shoot of extant species with penultimate branchlets. (E) Longitudinal section of penultimate branchlet showing one resin canal per each leaf (arrows), TX-70A-1. (F) Compound cladode-like segment of the branchlet junction showing resin canals, TX-35–1. (G) Ultimate shoot, TX-2–2. (H) Lower side of shoot showing stomatal bands. Scale bars for (A, G) = 2 mm, (B, C, E, H) = 3 mm, (D) = 5 mm (F) = 1 mm.
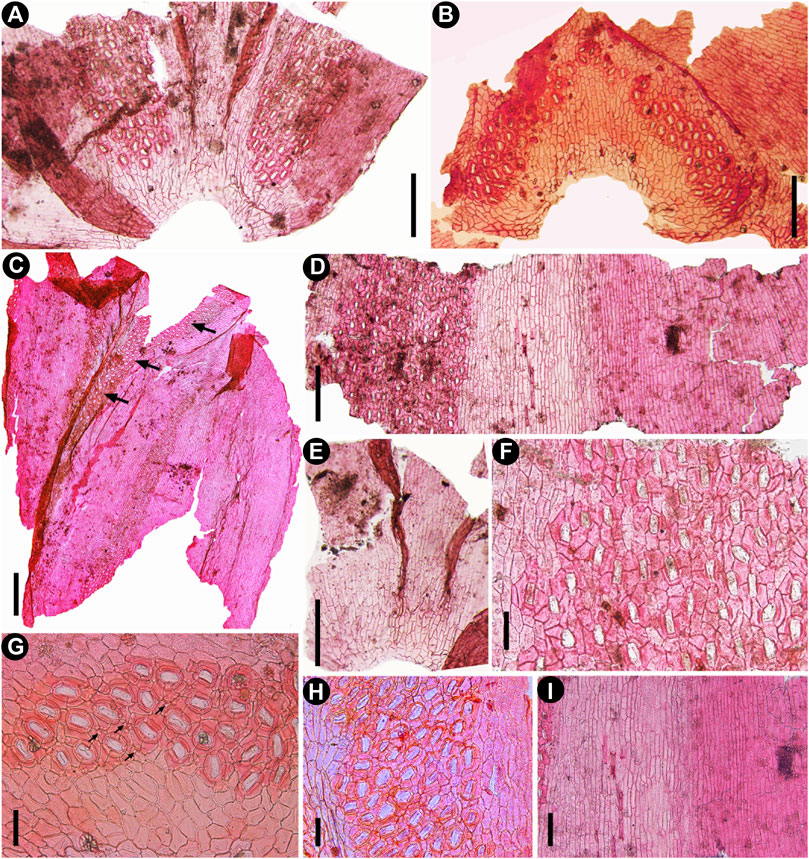
FIGURE 4. Cuticular characters of fossil Fokienia tianpingensis sp. nov., LM. (A) Cuticle of facial and two lateral leaves showing the distribution of stomatal bands on the underside of a branchlet, TX-63–2. (B) Stomatal distribution on the adaxial surface of a facial leaf of the shoot underside, TX-63–2. (C) The cuticle of one facial and one lateral leaves (from left to right) showing stomatal distribution on the abaxial and adaxial (arrow) surfaces, TX-39. (D) Abaxial cuticle of a lateral leaf showing the nonstomatal surface on the upper side of the branchlet (right) and a broad stomatal band on the underside of the branchlet (left), TX-34–2. (E) Narrow stomatal zones along the sutures between facial and lateral leaves on the upper side of a branchlet, TX-63–2. (F) Enlargement of the stomatal band in (D) showing the stomatal density and shared subsidiary cells. (G) Enlargement of stomata in (B) showing papillae within the stomatal band (arrows). (H) Enlargement of the stomatal band on the underside of the lateral leaf in (C). (I) Enlargement of nonstomatal zones of the lateral leaf in (D) on the upper side of branchlet (right) and on the underside (left). Scale bars for (A, B, D, E) = 200 μm, (F–H) = 400 μm, (I) = 100 μm.
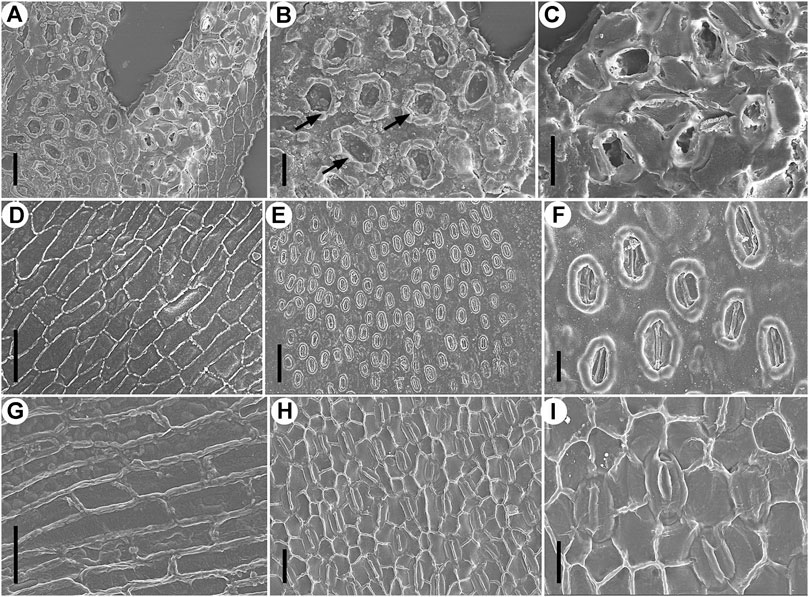
FIGURE 5. Epidermal characters of Fokienia tianpingensis sp. nov. (A–D) and extant Fokienia hodginsii (Dunn) Henry & Thomas (E–I), SEM. (A) Abaxial cuticle of a lateral leaf on the underside of a branchlet showing a stomatal band, outer (left) and inner (right) view, TX-63–2. (B) Enlargement of the stomatal band (outer view) in (A) showing Florin rings (arrows) and papillae. (C) Enlargement of stomata (inner view) in (A) sharing subsidiary cells. (D) Abaxial cuticle of a lateral leaf on the underside of a branchlet showing the morphology and arrangement of ordinary epidermal cells of a non-stomatal zone, inner view, TX-63–2. (E) Abaxial surface of a lateral leaf on the underside of a branchlet, outer view. (F) Enlargement of stomata in (E). (G) Abaxial cuticle of a lateral leaf on the underside of a branchlet showing the morphology and arrangement of ordinary epidermal cells of a non-stomatal zone, inner view. (H) Abaxial cuticle of a lateral leaf on the underside of a branchlet showing a stomatal band, inner view. (I) Enlargement of stomata in (H) sharing subsidiary cells. Scale bars for (A, D, G, H) = 40 μm, (B, C, F, I) = 20 μm.
Etymology
The epithet “tianpingensis” refers to the town near the fossil locality.
Holotype
TX-2-1, leafy shoot, designated here (Figure 3A).
Paratypes
TX-2-2, TX-34–2, TX-35–1, TX-38–1, TX-39, TX-63–2, TX-70A-1.
Material
Eight leafy shoots and isolated leaves from the Miocene Erzitang Formation in the Guiping Basin; type locality near Tianping Town, Tengxian County, Guangxi, South China.
Diagnosis
Foliage shoots alternately branching in the same plane. Leaves dimorphic, in whorls of four. Facial and lateral leaves almost equal in length. Leaves amphistomatic, stomata in broad bands on underside of foliage shoots. Facial leaves with a few stomata, lateral leaves nonstomatal on the upper side of shoots. Adaxial surface of facial leaves with stomatal bands at the leaf apex. Anticlinal walls of ordinary epidermal cells straight, periclinal walls slightly granular. Stomata monocyclic, rarely incompletely amphicyclic, densely spaced, longitudinally oriented, with 4-6 subsidiary cells, sharing 1-2 subsidiary cells. Florin rings prominent. Stomatal zones papillate.
Description
Foliage comprising three preserved branching orders are flattened in the same plane, the ultimate branchlets arise from the axil of lateral leaves of the penultimate branchlets in an alternate manner (Figures 3A,C,E). The incompletely preserved shoot bearing penultimate branchlets is 1.8 cm long, weakly flattened and narrower, but thicker than the penultimate branchlets (Figure 3C). Vegetative branchlets are convex on the upper side (Figures 3A,G) and concave on the lower side (Figure 3F). Leaves are scale-like, decussate, in whorls of four, strongly dimorphic, differentiated into laterals and facials (Figures 3A,C,E,G). The leaf base is decurrent, partly concealed by leaves of the lower pair (Figures 3A,C,G). Two lateral and two facial leaves constitute wedge-shaped, cladode-like segments stacked along branch axis. Each scale-like leaf has one resin canal (Figures 3E,F).
The lateral leaves are conduplicate, strongly bilaterally flattened and boat-shaped in surface view, 1.6–6.0 mm long (Figures 3A,E-G), but lateral leaves from penultimate shoots are smaller, 3.0–4.1 mm in length (Figure 3C). The margins of lateral leaves are entire. The apices of lateral leaves are acute or acuminate, slightly incurved, with free tips (Figures 3A,F,G). The facial leaves are dorsiventrally flattened and closely appressed to the shoot axis. They are wedge-shaped oblanceolate in outline, with obtuse apices, shorter than, or approximately equal in length to, the lateral leaves in the same whorl, up to 1.3–5.6 mm long (Figures 3A,F,G), but in the penultimate shoot facial leaves are shorter, 2.0–3.9 mm in length (Figure 3C).
The leaves are amphistomatic. The lateral leaves are nonstomatal on the upper side of foliage shoots (Figures 4C,D). The epidermal cells are elongated along the longitudinal cell files, more or less rectangular in outline, often with oblique end walls (Figures 4A,C–E). The anticlinal walls are straight, with numerous pits (Figure 5D). The external cuticular surfaces are generally even, but some cavities are occasionally observed. The cavities are irregular in distribution and size, round in outline, with a thickened rim. The periclinal cell walls are smooth to finely granular (Figure 5D).
The abaxial leaf surface on the underside of shoots displays one broad median stomatal zone and two narrow lateral nonstomatal zones (Figures 4A,C,D). The morphology and arrangement of ordinary epidermal cells of nonstomatal zones are similar to those of the upper side. The stomatal zone is strongly papillate, running along the leaf margin from the base to the apical region, but is absent from the leaf apex. The stomata are irregularly arranged, mostly oriented longitudinally (Figure 4F). Adjacent stomata sharing 1–2 subsidiary cells (Figure 4F).
The adaxial surface of the lateral leaf has one lateral stomatal zone on the underside of the foliage shoots (Figure 4C). Nonstomatal zones on each side of the stomatal zone only consist of a few files of ordinary epidermal cells, which are similar to those of the abaxial surface in morphology and arrangement (Figure 4C). A wide stomatal zone runs approximately the whole length of the leaf from the base, and stomata are closely spaced (Figure 4C).
The abaxial surfaces of facial leaves on the upper side of the shoots are generally nonstomatal with a few stomata running along the sutures between the facial and lateral leaves (Figure 4E). The epidermal cells are arranged in longitudinal files. The abaxial surfaces of facial leaves on the underside of foliage shoots possess broad median nonstomatal zones and two narrow lateral stomatal zones. The epidermal cells of nonstomatal zones are morphologically similar to those of the lateral leaves. The stomatal zones run along the sutures between the facial and lateral leaves (Figures 4A,C). The adaxial surfaces of facial leaves on both upper and lower sides of the shoots have two stomatal zones at the leaf apex running along the leaf margins (Figure 4B).
The stomatal complexes are monocyclic or very rarely incompletely amphicyclic, elliptical or sometimes elongate in shape (Figures 4F–H; Figures 5A,B). Stomata are densely spaced, generally longitudinally oriented, surrounded by 4–6 subsidiary cells, and adjacent stomata share 1–2 subsidiary cells (Figures 4D,F–H; Figures 5C). The subsidiary cells are polygonal, isodiametric or elongate in shape, and papillate (Figures 5A–C). The guard cells are slightly sunken, forming a usually closed aperture (Figure 5B). Florin rings are prominent, round or elliptical in outline (Figures 4G,H; Figures 5A,B).
Discussion
Comparison With Extant Genera
In gymnosperms, members of the Podocarpaceae and Cupressaceae possess scale leaves, but only in the Cupressoideae and Callitroideae subfamilies some genera produce branching in a single plane shoots with flattened whorled leaves (De Laubenfels, 1953). All genera of the subfamily Callitroideae are distributed in the Southern Hemisphere (Li, 1953; Li and Yang, 2002). The leaves of many genera within this subfamily, such as Fitzroya Hook. f., Actinostrobus Miq. and Callitris Vent., are arranged in alternating whorls of three, which are different from the leaves of our new fossil species from Tengxian in which leaves are arranged in whorls of four. The leaves of the new species form decussate whorls, whereas those of Widdringtonia Endl., Diselma Hook. f. and Papuacedrus H. L. Li are arranged in opposite decussate pairs (Farjon, 2005). The facial leaves of Papuacedrus, Austrocedrus Florin et Boutelje and Libocedrus Endl. are much smaller than their lateral leaves (Vidaković, 1991; Farjon, 2005), but the facial leaves of the present fossil are shorter or nearly equal to the lateral leaves. By these characters, new fossil species can be easily distinguished from the Callitroideae conifers.
The subfamily Cupressoideae comprises ten genera, including Chamaecyparis Spach, Platycladus Spach, Calocedrus Kurz, Thujopsis Siebold et Zucc. ex Endl., Cupressus Gray, Fokienia A. Henry et H. H. Thomas, Juniperus L., Microbiota Kom., Thuja L. and Tetraclinis Mast. (Li, 1953; Gadek et al., 2000; Li and Yang, 2002; Farjon, 2005). Although all the above genera and new fossils possess scale-like and whorled leaves, they can be easily distinguished from each other in macromorphological and cuticle characters, such as stomatal distribution, leaf shape and size. Among them, Thujopsis resembles the present fossil in having alternate branching, flattened ultimate shoots and dimorphic foliage, but differs in having obtuse apices on its facial and lateral leaves, the obvious separation between lateral and facial leaves, larger leaves (about 4–7 mm) and nonstomatal upper sides of leaves (Shi et al., 2012). The genus Thuja is different from the present fossil in having obovate or rhombic facial leaves with one gland, stomata with a loose distribution and basically no shared subsidiary cells (Shi et al., 2012). Cupressus differs in having irregular leaf placement, obliquely pendulous branches, simple or weakly dimorphic leaves, and triangle to diamond-shaped facial leaves. Juniperus can readily be distinguished from the new fossil foliage by having branchlets usually arranged irregularly, not in flattened sprays, with non-dimorphic leaves in alternating opposite pairs or in whorls of three, rarely in whorls of four. In comparison with the present fossil, the genus Calocedrus differs in posessing a serrate margin of lateral leaves, and loosely-spaced stomata that rarely share subsidiary cells (Shi et al., 2012). In foliage gross morphology, the present fossils are also obviously different from most other genera of the Cupressoideae. The facial leaves of Chamaecyparis are ovate or rhomboid-ovate; leaves of Platycladus are generally less than 3 mm; the facial leaves of Tetraclinis are linear and lateral leaves are long spoon shaped; and the branches of Microbiota are scattered, spreading, sagging and extending downward (Little et al., 2004; Farjon, 2005). The genus Fokienia is characterized by alternately branched flattened shoots, lateral leaves with entire margins, dense stomatal distribution and the stomata sharing subsidiary cells.
The new fossil species closely resembles the extant species of Fokienia in gross foliage morphology and epidermal characters. Both species have alternately branched shoots strongly flattened in the same plane, dimorphic decussate leaves in whorls of four, boat-shape lateral leaves with entire margins (Figure 3), papillate stomatal bands on abaxial surfaces of lateral and facial leaves on the lower side of shoots, but rarely on the adaxial surfaces of lateral leaves, as well as on the adaxial surface of facial leaves of both shoot sides, as well as monocyclic or very rarely incompletely amphicyclic stomata with distinct Florin rings, which are closely spaced and share subsidiary cells (Figures 4F–H; Figures 5A–C E,F,H,I; Figure 6) (Table 1). However, the facial and lateral leaves of the present fossils are smaller than those of the extant species, and the periclinal walls of the ordinary epidermal cells are slightly granular, while those of extant species are smooth (Figure 5G).
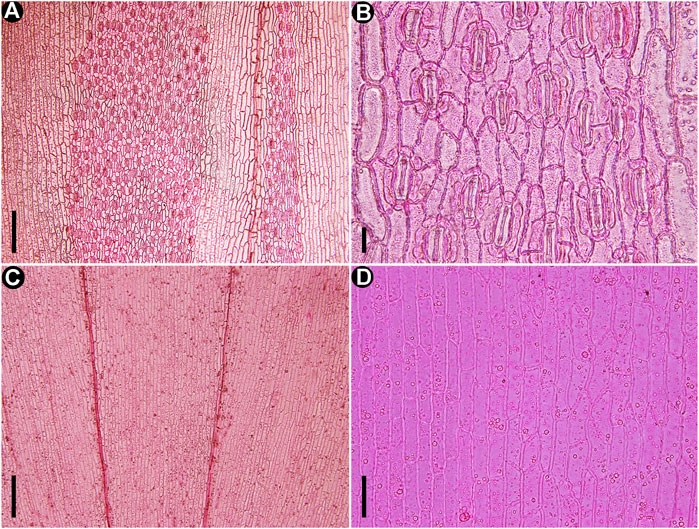
FIGURE 6. Cuticles of extant Fokienia hodginsii (Dunn) Henry et Thomas, LM. (A) The stomatal distribution on the abaxial surfaces of lateral (left) and facial (right) leaves on the underside of shoot. (B) Enlargement of lateral leaf stomatal band in (A) showing densely arranged stomata sharing subsidiary cells. (C) Cuticles of lateral and facial leaves on the upper surface of a shoot. (D) Enlargement of the epidermal cells in (C). Scale bars for (A, C) = 200 μm, (B) = 20 μm, (D) = 50 μm.
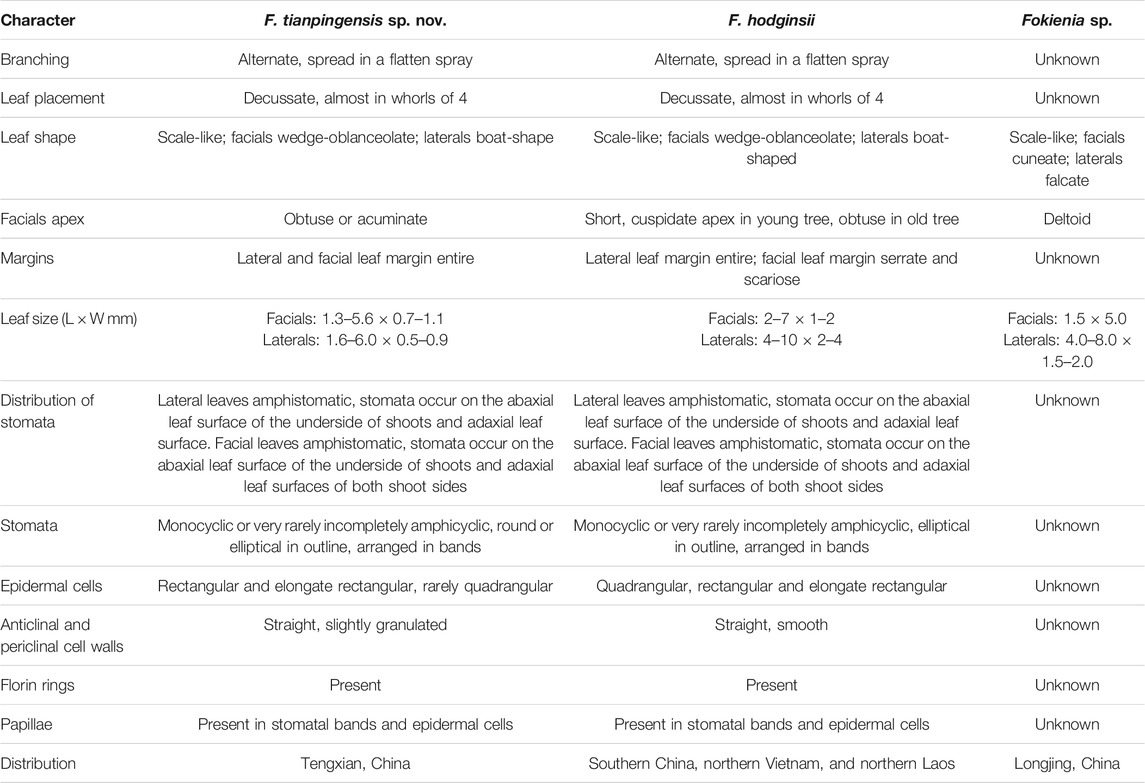
TABLE 1. Comparison of Fokienia tianpingensis sp. nov. with another fossil species and extant Fokienia hodginsii.
Comparison With Fossil Species of Fokienia
Fossils of Fokienia were rarely found in the past. Fokienia shengxianensis He, Sun & Liu from the Miocene of Zhejiang (He et al., 2012) was reassigned to Calocedrus shengxianensis (He, Sun & Liu) Zhang & Zhou (Zhang et al., 2015) based on sparsely spaced stomata that rarely share subsidiary cells. Fokienia catenulata (Bell)R.W. Brown and Fokienia ravenscragensis McIver & Basinger were revised to the extinct genus Ditaxocladus based on the oppositely branched leaf sprays and oppositely clustered seed cones with much fewer cone scales (Brown, 1962; Mciver and Basinger, 1990; Mciver, 1992; Guo et al., 2012). Fokienia sp. from the Oligocene of Loingjing, Jilin, is similar to the new extinct species in leaf morphology, but its epidermal characters are unknown. The facial leaves of the present fossils are wedge-oblanceolate with obtuse or acuminate apices and the lateral leaves are boat-shaped, while the facial leaves of Fokienia sp. are wedge-shaped with a deltoid apex and its lateral leaves are falcate. The facial leaves of the present fossil and those of Fokienia sp. are similar in size but the lateral leaves of the former are much smaller than those of the latter.
Palaeogeographic and Palaeoecological Implications
Available palaeobotanical evidence indicates that the oldest known fossil record of Fokienia is from the Oligocene of Longjing, Jilin, China (Figure 1; Guo and Zhang, 2002). Combined with the previous phylogenetic study of Cupressaceae (Mao et al., 2012; Qu et al., 2017) and fossil records, Yin et al. (2020) studied the phylogeography of F. hodginsii based on the molecular markers of chloroplast and single-copy nuclear genes. This analysis revealed that F. hodginsii comprised two major phylogeographic lineages located on either side of the Tanaka-Kaiyong Line, which is considered to be a major phytogeographic boundary in southwest China, separating Sino-Japanese and Sino-Himalayan floristic subkingdoms of East Asia (Li and Li, 1997; Yin et al., 2020). The divergence time of these two major lineages was dated to the early Miocene (ca. 19.34–19.95 Ma) (Yin et al., 2020). Yin et al. (2020) speculated the ancestors of Fokienia migrated from the middle latitudes of the Northern Hemisphere to the subtropical regions after the formation of the East Asian monsoon. In addition, combined with the obvious phylogeographic structure and ENM results, it seems the F. hodginsii survived in four refuges during the last glacial episodes, which is consistent with the multiple refugia patterns of relict species in subtropical China (Yin et al., 2020). The F. hodginsii population has a relatively high genetic diversity, which may be related to its long history in a variety of geographical habitats (Yin et al., 2018, Yin et al., 2020). However, the fossils (F. shengxianensis He, Sun & Liu, F. catenulata (Bell) R.W. Brown and F. ravenscragensis McIver & Basinger) used for the fossil calibration points have been reassigned to other genera. Consequently, the time to the most recent common ancestors of intraspecific lineages in F. hodginsii and phytogeography of the genus should be reconsidered. Now the fossil record of Fokienia is enriched by Fokienia tianpingensis sp. nov. from the Miocene Erzitang Formation, Tengxian, Guangxi. From the present two fossil records, we infer that Fokienia already existed in middle latitudes of the Northern Hemisphere at least as early as the Oligocene, as global climate cooling gradually drove it to extinction in the mid-latitudes and migrated southward to South China in the Miocene (Figure 1), which is consistent with the above phylogeographical context.
Extant Fokienia is generally a minor constituent of subtropical to warm temperate evergreen (mixed) mesophytic forest. This genus forms nearly pure stands at elevations of 350–2,300 m on slopes and ridges of mountains with soils derived from both granite and limestone or is associated with other conifers (e.g., Dacrydium, Cunninghamia, Nothotsuga, Cephalotaxus) and broad-leaved tree species of the families Fagaceae, Lauraceae, Theaceae and Magnoliaceae (Farjon, 2005, 2017). Being not cold-tolerant, Fokienia prefers a humid and warm climate, with precipitation in excess of 1,200 mm and a mean annual temperature range of 15.4–23.6°C. It rarely grows in frost-prone areas (Farjon, 2005; Tam et al., 2011; Huang et al., 2013).
Apart from Fokienia, a large number of fossils of gymnosperms and angiosperms have been found in Guiping Basin, e.g., representatives of Pinaceae (Pinus L., Keteleeria Carr.), Podocarpaceae (Dacrycarpus (Endl.) de Laub.), Burseraceae (Canarium Linn.), Fagaceae and Theaceae (Han et al., 2018; Wu et al., 2021). Among them, Dacrycarpus is a robust indicator for a very wet palaeoenvironment, as it requires high precipitation and is one of the least drought-tolerant genera, and is often distributed in tropical mountains. According to the taxonomic composition of the fossil flora and the niches of related modern species, it can be inferred that in the Miocene the Guiping Basin was covered in an evergreen broad-coniferous mixed forest growing under warm and humid climatic conditions.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
JJ, CQ, TK, and NM designed the research. XW, JJ, and SJ collected fossil and modern leaves. XW, HZ, and SJ prepared and studied the cuticles with LM and SEM. JJ, XW, QY, TK, and NM analyzed and interpreted the results. All authors contributed feedback on drafts, revised and approved the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 42072020, 42111530024, 41820104002, 41572011), the China Postdoctoral Science Foundation (2021M693589), the Fundamental Research Funds for the Central Universities (2021qntd18), the Russian Foundation for Basic Research (RFBR, Grant No. 21-54-53001, to NM), and the State Program (No. 0135-2019-0045, Geological Institute, Russian Academy of Sciences, to TK).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We greatly thank Gongle Shi from Nanjing Institute of Geology and Paleontology, Chinese Academy of Sciences (NIGPAS) for his helpful suggestions. We also express sincere gratitude to graduate students majoring in botany at Sun Yat-sen University for their assistance in field work. We are very grateful to Robert Spicer from The Open University, United Kingdom, and Teresa Spicer for their language help. Our deepest gratitude goes to the editor and reviewers for their careful work and thoughtful suggestions.
References
Brown, R. W. (1962). Paleocene Flora of the Rocky Mountains and Great Plains. U. S. Geol. Surv. Prof. Paper. 375, 50–51.
Duka, I. (2015). DigicamControl: Free Windows DSLR Camera Controlling Solution. Available at: http://digicamcontrol.com/ (Accessed May 13, 2021).
Dunn, S. T. (1908). A Botanical Expedition to Central Fokien. J. Linn. Soc. Bot. 38, 350–373. doi:10.1111/j.1095-8339.1908.tb00858.x
Farjon, A. (2017). A Handbook of the World’s Conifers. 2nd edition. Leiden, Netherlands: Brill Academic Publishers. doi:10.1163/9789004324510
Fu, L. G., Yu, Y., Adams, R. P., and Farjon, A. (1999). “Cupressaceae”, in Flora of China,” in Cycadaceae through Fagaceae. Editors Z. Y. Wu, and P. H. Raven (Beijing, China: Science Press, Beijing, and Missouri Botanical Garden Press), Vol. 4, 62–77.
Gadek, P. A., Alpers, D. L., Heslewood, M. M., and Quinn, C. J. (2000). Relationships within Cupressaceae Sensu Lato: a Combined Morphological and Molecular Approach. Am. J. Bot. 87, 1044–1057. doi:10.2307/2657004
Gadek, P. A., and Quinn, C. J. (1993). An Analysis of Relationships within the Cupressaceae Sensu Stricto Based on rbcL Sequences. Ann. Mo. Bot. Garden 80, 581–586. doi:10.2307/2399847
Guo, S. X., Kvaček, Z., Manchester, S. R., and Zhou, Z. K. (2012). Ditaxocladus (Extinct Cupressaceae, Cupressoideae) from the Upper Cretaceous and Paleocene of the Northern Hemisphere. Palaeontogr. Abt B. 288, 135–159. doi:10.1127/palb/288/2012/135
Guo, S. X., and Zhang, G. F. (2002). Oligocene Sanhe flora in Longjing County of Jilin, Northeast China. Acta Palaeontol. Sin. 2, 193–210.
Han, M., Manchester, S. R., Wu, Y., Jin, J., and Quan, C. (2018). Fossil Fruits of Canarium (Burseraceae) from Eastern Asia and Their Implications for Phytogeographical History. J. Syst. Palaeontology 16, 841–852. doi:10.1080/14772019.2017.1349624
He, W., Sun, B., and Liu, Y. S. (2012). Fokienia Shengxianensis Sp. Nov. (Cupressaceae) from the Late Miocene of Eastern China and its Paleoecological Implications. Rev. Palaeobotany Palynology 176-177, 24–34. doi:10.1016/j.revpalbo.2012.03.013
Huang, S. J., Rong, J. D., Zhang, L. H., Yang, Y., Jiang, J. L., and Zheng, Y. S. (2013). Research Summarization of Fokienia Hodgirtsii. J. Fujian For. Sci. Tech. 8, 319.
Jagel, A., and Dörken, V. M. (2015). Morphology and Morphogenesis of the Seed Cones of the Cupressaceae - Part II. Cupressoideae. Bull CCP, 4, 51–78.
Kvaček, Z., Manchester, S. R., and Schorn, H. E. (2000). Cones, Seeds, and Foliage of Tetraclinis Salicornioides (Cupressaceae) from the Oligocene and Miocene of Western North America: a Geographic Extension of the European Tertiary Species. Int. J. Plant Sci. 161, 331–344. doi:10.1086/314245
Kvaček, Z., and Rember, W. C. (2007). Calocedrus Robustior (Cupressaceae) and Taxus Schornii (Taxaceae): Two New Conifers from the Middle Miocene Latah Formation of Northern Idaho. PaleoBios 27, 68–79.
Li, C. X., and Yang, Q. (2002). Phylogenetic Relationships Among the Genera of Taxodiaceae and Cupressaceae. Life Sci. Res. 6, 56–60.
Li, H. L. (1953). Present Distribution and Habitats of the Conifers and Taxads. Evolution 7, 245–261. doi:10.1111/j.1558-5646.1953.tb00086.x
Li, X. W., and Li, J. (1997). The Tanaka–Kaiyong Line – an Important Floristic Line for the Study of the flora of East Asia. Ann. Mo. Bot. Gard. 84, 888–892.
Lin, Z. C. (2007). Terrestrial Fossil Plants of the Mesozoic and the Cenozoic from West Hebei and West Yunnan and Palaeoatmospheric CO2 Reconstruction. Lanzhou, Gansu, China: Lanzhou University. [dissertation’s thesis].
Little, D. P., Schwarzbach, A. E., Adams, R. P., and Hsieh, C. F. (2004). The Circumscription and Phylogenetic Relationships of Callitropsis and the Newly Described Genus Xanthocyparis (Cupressaceae). Am. J. Bot. 91, 1872–1881. doi:10.3732/ajb.91.11.1872
Mao, K., Milne, R. I., Zhang, L., Peng, Y., Liu, J., Thomas, P., et al. (2012). Distribution of Living Cupressaceae Reflects the Breakup of Pangea. Proc. Natl. Acad. Sci. 109, 7793–7798. doi:10.1073/pnas.1114319109
Mciver, E. E., and Basinger, J. F. (1990). Fossil Seed Cones of Fokienia (Cupressaceae) from the Paleocene Ravenscrag Formation of Saskatchewan, Canada. Can. J. Bot. 68, 1609–1618. doi:10.1139/b90-207
Mciver, E. E. (1992). Fossil Fokienia (Cupressaceae) from the Paleocene of Alberta, Canada. Can. J. Bot. 70, 742–749. doi:10.1139/b92-095
Qu, X. J., Jin, J. J., Chaw, S. M., Li, D. Z., and Yi, T. S. (2017). Multiple Measures Could Alleviate Long–branch Attraction in Phylogenomic Reconstruction of Cupressoideae (Cupressaceae). Sci. Rep. 7, 41005. doi:10.1038/srep41005
Rushforth, K. D. (2007). Notes on the Cupressaceae in Vietnam. Vietnam. J. Bio. 29, 32–39. doi:10.15625/0866-7160/v29n3.5384
Shi, G., Zhou, Z., and Xie, Z. (2012). A New Oligocene Calocedrus from South China and its Implications for Transpacific Floristic Exchanges. Am. J. Bot. 99, 108–120. doi:10.3732/ajb.1100331
Shi, G., Zhou, Z., and Xie, Z. (2011). Cupressus Foliage Shoots and Associated Seed Cones from the Oligocene Ningming Formation of Guangxi, South China. Rev. Palaeobotany Palynology 166, 325–334. doi:10.1016/j.revpalbo.2011.06.005
Tam, M. N., Trang, N. T. P., and Hoa, N. T. (2011). Genetic Diversity of an Endangered Species, Fokienia Hodginsii (Cupressaceae). Afr. J. Biotech. 10, 15838–15844. doi:10.5897/AJB10.2299
Wang, J. Z. (2007). Anatomical Studies on Stems and Leaves of 14 Species of Taxodiaceae and Cupressaceae. Nanning, Guangxi: Guangxi University. [dissertation’s thesis]. [Guangxi].
Wu, J. Y., Ding, S. T., Li, Q. J., Zhao, Z. R., and Sun, B. N. (2014). First Occurrence of Platycladus from the Upper Miocene of Southwest China and its Phytogeographic Implications. PLoS ONE 9, e115141. doi:10.1371/journal.pone.0115141
Wu, X. K., Zavialova, N. E., Kodrul, T. M., Liu, X. Y., Gordenko, N. V., Maslova, N. P., et al. (2021). Northern Hemisphere Megafossil of Dacrycarpus (Podocarpaceae) from the Miocene of South China and its Evolutionary and Paleoecological Implications. J. Syst. Evol. 59, 352–374. doi:10.1111/jse.12534
Wu, Y., Jin, J. H., Li, N., He, H. M., Chen, T., and Liu, X. Y. (2019). Early Oligocene Calocedrus (Cupressaceae) from the Maoming Basin, South China, and its Paleogeographic and Paleoclimatic Implications. Jnl Sytematics Evol. 57, 142–152. doi:10.1111/jse.12424
Yang, Z. Y., Ran, J. H., and Wang, X. Q. (2012). Three Genome-Based Phylogeny of Cupressaceae s.l.: Further Evidence for the Evolution of Gymnosperms and Southern Hemisphere Biogeography. Mol. Phylogenet. Evol. 64, 452–470. doi:10.1016/j.ympev.2012.05.004
Ye, M. N. (1981). “On the Preparation Methods of Fossil Cuticle,” in Selected Papers of the 12th Annual Conference of the Palaeontological Society of China (Beijing: Beijing: Science Press), 170–179.
Yin, Q., Chen, S., Guo, W., Huang, Y., Huang, Y., Zhou, R., et al. (2018). Pronounced Genetic Differentiation in Fokienia Hodginsii Revealed by Simple Sequence Repeat Markers. Ecol. Evol. 8, 10938–10951. doi:10.1002/ece3.4560
Yin, Q. Y., Fan, Q., Li, P., Truong, D., Zhao, W. Y., Zhou, R. C., et al. (2020). Neogene and Quaternary Climate Changes Shaped the Lineage Differentiation and Demographic History of Fokienia Hodginsii (Cupressaceae s.l.), a Tertiary Relict in East Asia. J. Syst. Evol. doi:10.1111/jse.12582
Zhang, J. W., Huang, J., D'Rozario, A., Adams, J. M., and Zhou, Z. K. (2015). Calocedrus Shengxianensis, a Late Miocene Relative of C. Macrolepis (Cupressaceae) from South China: Implications for Paleoclimate and Evolution of the Genus. Rev. Palaeobotany Palynology 222, 1–15. doi:10.1016/j.revpalbo.2015.07.004
Keywords: Miocene, conifers, leaf epidermal characters, Fokienia, Cupressaceae
Citation: Wu X, Zhang H, Kodrul TM, Maslova NP, Jiang S, Yin Q, Quan C and Jin J (2021) First Fossil Fokienia (Cupressaceae) in South China and Its Palaeogeographic and Palaeoecological Implications. Front. Earth Sci. 9:709663. doi: 10.3389/feart.2021.709663
Received: 14 May 2021; Accepted: 22 June 2021;
Published: 05 July 2021.
Edited by:
Haijun Song, China University of Geosciences Wuhan, ChinaCopyright © 2021 Wu, Zhang, Kodrul, Maslova, Jiang, Yin, Quan and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Jin, lssjjh@mail.sysu.edu.cn; Cheng Quan, quan@chd.edu.cn
 Xinkai Wu1
Xinkai Wu1  Hao Zhang
Hao Zhang Tatiana M. Kodrul
Tatiana M. Kodrul Jianhua Jin
Jianhua Jin