Do Different Tree-Ring Proxies Contain Different Temperature Signals? A Case Study of Norway Spruce (Picea abies (L.) Karst) in the Eastern Carpathians
Abstract
:1. Introduction
- How does air temperature modulate Norway spruce growth in an intramountain valley of the Carpathians?
- Is the correlation between temperature and the investigated tree-ring parameters stable through time?
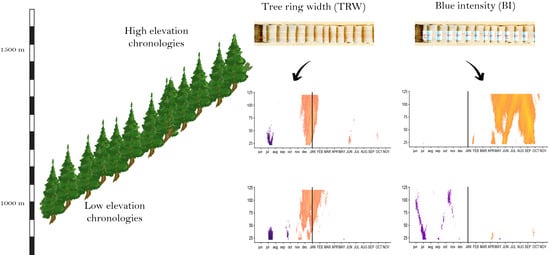
2. Results and Discussion
2.1. Description of Chronologies
2.2. Climate–Growth Relationships for Three Tree-Ring Parameters
2.3. Time Stability of Climate–Growth Relationship
3. Materials and Methods
3.1. Study Area
3.2. Sample Collection and Data Processing
3.3. Climatic Dataset
3.4. Climate–Growth Relationship Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fritts, H. Tree Rings and Climate; Elsevier: Amsterdam, The Netherlands, 1976; ISBN 0323145280. [Google Scholar]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010; ISBN 0816526842. [Google Scholar]
- Kramer, K.; Leinonen, I.; Loustau, D. The importance of phenology for the evaluation of impact of climate change on growth of boreal, temperate and Mediterranean forests ecosystems: An overview. Int. J. Biometeorol. 2000, 44, 67–75. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- European Environment Agency. European Forest Ecosystems: State and Trends; Publications Office of European Union: Luxembourg, 2016. [Google Scholar]
- Bigler, C.; Veblen, T.T. Increased early growth rates decrease longevities of conifers in subalpine forests. Oikos 2009, 118, 1130–1138. [Google Scholar] [CrossRef]
- Di Filippo, A.; Pederson, N.; Baliva, M.; Brunetti, M.; Dinella, A.; Kitamura, K.; Knapp, H.D.; Schirone, B.; Piovesan, G. The longevity of broadleaf deciduous trees in Northern Hemisphere temperate forests: Insights from tree-ring series. Front. Ecol. Evol. 2015, 3, 46. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Franklin, J.F. Conserving Forest Biodiversity: A Comprehensive Multiscaled Approach; Island Press: Washington, DC, USA, 2002; ISBN 1559639350. [Google Scholar]
- Pretzsch, H.; Biber, P.; Schütze, G.; Uhl, E.; Rötzer, T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 2014, 5, 4967. [Google Scholar] [CrossRef]
- Vitali, V.; Büntgen, U.; Bauhus, J. Seasonality matters—The effects of past and projected seasonal climate change on the growth of native and exotic conifer species in Central Europe. Dendrochronologia 2018, 48, 1–9. [Google Scholar] [CrossRef]
- Parobeková, Z.; Sedmáková, D.; Kucbel, S.; Pittner, J.; Jaloviar, P.; Saniga, M.; Balanda, M.; Vencurik, J. Influence of disturbances and climate on high-mountain Norway spruce forests in the Low Tatra Mts., Slovakia. For. Ecol. Manag. 2016, 380, 128–138. [Google Scholar] [CrossRef]
- Jones, P.D.; Briffa, K.R.; Osborn, T.J.; Lough, J.M.; van Ommen, T.D.; Vinther, B.M.; Luterbacher, J.; Wahl, E.R.; Zwiers, F.W.; Mann, M.E.; et al. High-resolution palaeoclimatology of the last millennium: A review of current status and future prospects. Holocene 2009, 19, 3–49. [Google Scholar] [CrossRef]
- Büntgen, U.; Frank, D.C.; Nievergelt, D.; Esper, J. Summer Temperature Variations in the European Alps, a.d. 755–2004. J. Clim. 2006, 19, 5606–5623. [Google Scholar] [CrossRef]
- Schweingruber, F.; Fritts, H.; Bräker, O.; Drew, L.; Schär, E. The X-ray technique as applied to dendroclimatology. Tree Ring Bull. 1978, 38, 61–91. [Google Scholar]
- Wilson, R.; Anchukaitis, K.; Briffa, K.R.; Büntgen, U.; Cook, E.; D’Arrigo, R.; Davi, N.; Esper, J.; Frank, D.; Gunnarson, B.; et al. Last millennium northern hemisphere summer temperatures from tree rings: Part I: The long term context. Quat. Sci. Rev. 2016, 134, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Nagavciuc, V.; Roibu, C.-C.; Ionita, M.; Mursa, A.; Cotos, M.-G.; Popa, I. Different climate response of three tree ring proxies of Pinus sylvestris from the Eastern Carpathians, Romania. Dendrochronologia 2019, 54, 56–63. [Google Scholar] [CrossRef]
- Nagavciuc, V.; Kern, Z.; Ionita, M.; Hartl, C.; Konter, O.; Esper, J.; Popa, I. Climate signals in carbon and oxygen isotope ratios of Pinus cembra tree-ring cellulose from the Călimani Mountains, Romania. Int. J. Clim. 2020, 40, 2539–2556. [Google Scholar] [CrossRef]
- Koprowski, M.; Duncker, P. Tree ring width and wood density as the indicators of climatic factors and insect outbreaks affecting spruce growth. Ecol. Indic. 2012, 23, 332–337. [Google Scholar] [CrossRef]
- McCarroll, D.; Pettigrew, E.; Luckman, A.; Guibal, F.; Edouard, J.-L. Blue Reflectance Provides a Surrogate for Latewood Density of High-latitude Pine Tree Rings. Arct. Antarct. Alp. Res. 2002, 34, 450–453. [Google Scholar] [CrossRef]
- Campbell, R.; McCarroll, D.; Loader, N.J.; Grudd, H.; Robertson, I.; Jalkanen, R. Blue intensity in Pinus sylvestris tree-rings: Developing a new palaeoclimate proxy. Holocene 2007, 17, 821–828. [Google Scholar] [CrossRef]
- Rydval, M.; Larsson, L.-Å.; McGlynn, L.; Gunnarson, B.E.; Loader, N.J.; Young, G.H.F.; Wilson, R. Blue intensity for dendroclimatology: Should we have the blues? Experiments from Scotland. Dendrochronologia 2014, 32, 191–204. [Google Scholar] [CrossRef]
- Björklund, J.A.; Gunnarson, B.E.; Seftigen, K.; Esper, J.; Linderholm, H.W. Blue intensity and density from northern Fennoscandian tree rings, exploring the potential to improve summer temperature reconstructions with earlywood information. Clim. Past 2014, 10, 877–885. [Google Scholar] [CrossRef]
- Campbell, R.; McCarroll, D.; Robertson, I.; Loader, N.J.; Grudd, H.; Gunnarson, B. Blue Intensity In Pinus sylvestris Tree Rings: A Manual for A New Palaeoclimate Proxy. Tree-Ring Res. 2011, 67, 127–134. [Google Scholar] [CrossRef]
- Wilson, R.; Rao, R.; Rydval, M.; Wood, C.; Larsson, L.-Å.; Luckman, B.H. Blue Intensity for dendroclimatology: The BC blues: A case study from British Columbia, Canada. Holocene 2014, 24, 1428–1438. [Google Scholar] [CrossRef]
- Björklund, J.A.; Gunnarson, B.E.; Seftigen, K.; Esper, J.; Linderholm, H.W. Is Blue Intensity Ready to Replace Maximum Latewood Density as a Strong Temperature Proxy? A Tree-Ring Case Study on Scots Pine from Northern Sweden. Clim. Past Discuss. 2013, 9, 5227–5261. [Google Scholar] [CrossRef]
- Biondi, F. Comparing tree-ring chronologies and repeated timber inventories as forest monitoring tools. Ecol. Appl. 1999, 9, 12. [Google Scholar] [CrossRef]
- West, P. Use of diameter increment and basal area increment in tree growth studies. Can. J. For. Res. 1980, 10, 71–77. [Google Scholar] [CrossRef]
- Biondi, F.; Qeadan, F. A Theory-Driven Approach to Tree-Ring Standardization: Defining the Biological Trend from Expected Basal Area Increment. Tree-Ring Res. 2008, 64, 81–96. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Liu, B.; Huang, R.; Camarero, J.J. Moisture mediates temperature-growth couplings of high-elevation shrubs in the Tibetan plateau. Trees 2022, 36, 273–281. [Google Scholar] [CrossRef]
- Caudullo, G.; Tinner, W.; de Rigo, D. Picea abies in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Publication Office of EU: Luxembourg, 2016. [Google Scholar]
- Klimo, E.; Hager, H.; Kulhavý, J. Spruce Monocultures in Central Europe: Problems and Prospects; European Forest Institute Joensuu: Joensuu, Finland, 2000; Volume 33. [Google Scholar]
- Paquette, A.; Messier, C. The effect of biodiversity on tree productivity: From temperate to boreal forests: The effect of biodiversity on the productivity. Glob. Ecol. Biogeogr. 2011, 20, 170–180. [Google Scholar] [CrossRef]
- Schütz, J.-P.; Götz, M.; Schmid, W.; Mandallaz, D. Vulnerability of spruce (Picea abies) and beech (Fagus sylvatica) forest stands to storms and consequences for silviculture. Eur. J. For. Res 2006, 125, 291–302. [Google Scholar] [CrossRef]
- Bošel’a, M.; Sedmák, R.; Sedmáková, D.; Marušák, R.; Kulla, L. Temporal shifts of climate–growth relationships of Norway spruce as an indicator of health decline in the Beskids, Slovakia. For. Ecol. Manag. 2014, 325, 108–117. [Google Scholar] [CrossRef]
- Levanič, T.; Gričar, J.; Gagen, M.; Jalkanen, R.; Loader, N.J.; McCarroll, D.; Oven, P.; Robertson, I. The climate sensitivity of Norway spruce [Picea abies (L.) Karst.] in the southeastern European Alps. Trees 2009, 23, 169–180. [Google Scholar] [CrossRef]
- Mäkinen, H.; Nöjd, P.; Kahle, H.-P.; Neumann, U.; Tveite, B.; Mielikäinen, K.; Röhle, H.; Spiecker, H. Radial growth variation of Norway spruce (Picea abies (L.) Karst.) across latitudinal and altitudinal gradients in central and northern Europe. For. Ecol. Manag. 2002, 171, 243–259. [Google Scholar] [CrossRef]
- Ponocná, T.; Spyt, B.; Kaczka, R.; Büntgen, U.; Treml, V. Growth trends and climate responses of Norway spruce along elevational gradients in East-Central Europe. Trees 2016, 30, 1633–1646. [Google Scholar] [CrossRef]
- Savva, Y.; Oleksyn, J.; Reich, P.B.; Tjoelker, M.G.; Vaganov, E.A.; Modrzynski, J. Interannual growth response of Norway spruce to climate along an altitudinal gradient in the Tatra Mountains, Poland. Trees 2006, 20, 735–746. [Google Scholar] [CrossRef]
- Koprowski, M.; Zielski, A. Dendrochronology of Norway spruce (Picea abies (L.) Karst.) from two range centres in lowland Poland. Trees 2006, 20, 383–390. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Rathgeber, C.B.K.; Ulrich, E. Sensitivity of French temperate coniferous forests to climate variability and extreme events (Abies alba, Picea abies and Pinus sylvestris). J. Veg. Sci. 2010, 21, 364–376. [Google Scholar] [CrossRef]
- Pichler, P.; Oberhuber, W. Radial growth response of coniferous forest trees in an inner Alpine environment to heat-wave in 2003. For. Ecol. Manag. 2007, 242, 688–699. [Google Scholar] [CrossRef]
- Van der Maaten-Theunissen, M.; Kahle, H.-P.; van der Maaten, E. Drought sensitivity of Norway spruce is higher than that of silver fir along an altitudinal gradient in southwestern Germany. Ann. For. Sci. 2013, 70, 185–193. [Google Scholar] [CrossRef]
- Barry, R.G. Mountain Weather and Climate; Psychology Press: London, UK, 1992; ISBN 0415071135. [Google Scholar]
- Kolář, T.; Čermák, P.; Trnka, M.; Žid, T.; Rybníček, M. Temporal changes in the climate sensitivity of Norway spruce and European beech along an elevation gradient in Central Europe. Agric. For. Meteorol. 2017, 239, 24–33. [Google Scholar] [CrossRef]
- Begović, K.; Rydval, M.; Mikac, S.; Čupić, S.; Svobodova, K.; Mikoláš, M.; Kozák, D.; Kameniar, O.; Frankovič, M.; Pavlin, J.; et al. Climate-growth relationships of Norway Spruce and silver fir in primary forests of the Croatian Dinaric mountains. Agric. For. Meteorol. 2020, 288–289, 108000. [Google Scholar] [CrossRef]
- Kaczka, R.J.; Spyt, B.; Janecka, K.; Beil, I.; Büntgen, U.; Scharnweber, T.; Nievergelt, D.; Wilmking, M. Different maximum latewood density and blue intensity measurements techniques reveal similar results. Dendrochronologia 2018, 49, 94–101. [Google Scholar] [CrossRef]
- Tsvetanov, N.; Dolgova, E.; Panayotov, M. First measurements of Blue intensity from Pinus peuce and Pinus heldreichii tree rings and potential for climate reconstructions. Dendrochronologia 2020, 60, 125681. [Google Scholar] [CrossRef]
- Schwab, N.; Kaczka, R.; Janecka, K.; Böhner, J.; Chaudhary, R.; Scholten, T.; Schickhoff, U. Climate Change-Induced Shift of Tree Growth Sensitivity at a Central Himalayan Treeline Ecotone. Forests 2018, 9, 267. [Google Scholar] [CrossRef] [Green Version]
- Wigley, T.M.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Sidor, C.G.; Popa, I.; Vlad, R.; Cherubini, P. Different tree-ring responses of Norway spruce to air temperature across an altitudinal gradient in the Eastern Carpathians (Romania). Trees 2015, 29, 985–997. [Google Scholar] [CrossRef]
- Bouriaud, O.; Popa, I. Comparative dendroclimatic study of Scots pine, Norway spruce, and silver fir in the Vrancea Range, Eastern Carpathian Mountains. Trees 2009, 23, 95–106. [Google Scholar] [CrossRef]
- Leonelli, G.; Pelfini, M. Influence of climate and climate anomalies on norway spruce tree-ring growth at different altitudes and on glacier responses: Examples from the central italian alps. Geogr. Ann. Ser. A Phys. Geogr. 2008, 90, 75–86. [Google Scholar] [CrossRef]
- Apăvaloae, M.; Apostol, L.; Pîrvulescu, I. Inversiunile termice din culoarul Moldovei (sectorul Câmpulung Moldovenesc–Frasin) și influența lor asupra poluării atmosferei. Sci. Ann. “Stefan Cel Mare” Univ. Geogr. Ser. 1994, 5. [Google Scholar]
- Ahrens, C.D. Meteorology Today: An Introduction to Weather, Climate, and the Environment; Cengage Learning Canada, Inc.: Toronto, ON, Canada, 2015; ISBN 0176728333. [Google Scholar]
- Apăvaloae, M.; Pîrvulescu, I.; Apostol, L. Caracteristici ale inversiunilor termice din Podișul Fălticenilor. Lucr. Semin. Geogr. “Dimitrie Cantemir” 1987, 8. [Google Scholar]
- Ciutea, A.; Jitariu, V. Thermal inversions identification through the analysis of the vegetation inversions occurred in the forest ecosystems from the Eastern Carpathians. Present Environ. Sustain. Dev. 2020, 14, 29–42. [Google Scholar] [CrossRef]
- Ichim, P.; Apostol, L.; Sfîcă, L.; Kadhim-Abid, A.-L.; Istrate, V. Frequency of Thermal Inversions Between Siret and Prut Rivers in 2013. Present Environ. Sustain. Dev. 2014, 8, 267–284. [Google Scholar] [CrossRef]
- Sfîcă, L.; Nicuriuc, I.; Niță, A. Boundary Layer Temperature Stratification as Result of Atmospheric Circulation within the Western Side of Brașov Depression. In Proceedings of the 2019 Air and Water Components of the Environment Conference, Cluj-Napoca, Romania, 22–24 March 2019; pp. 53–64. [Google Scholar]
- Palffy, E. Temperature inversion in the Csik basin. Acta Clim. 1995, 28, 41–45. [Google Scholar]
- Mayr, S.; Schmid, P.; Beikircher, B.; Feng, F.; Badel, E. Die hard: Timberline conifers survive annual winter embolism. New Phytol. 2020, 226, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2007, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Rathgeber, C.B.K.; Deslauriers, A. Comparing needle and shoot phenology with xylem development on three conifer species in Italy. Ann. For. Sci. 2009, 66, 206. [Google Scholar] [CrossRef]
- Buntgen, U.; Frank, D.C.; Kaczka, R.J.; Verstege, A.; Zwijacz-Kozica, T.; Esper, J. Growth responses to climate in a multi-species tree-ring network in the Western Carpathian Tatra Mountains, Poland and Slovakia. Tree Physiol. 2007, 27, 689–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putalová, T.; Vacek, Z.; Vacek, S.; Štefančík, I.; Bulušek, D.; Král, J. Tree-ring widths as an indicator of air pollution stress and climate conditions in different Norway spruce forest stands in the Krkonoše Mts. Cent. Eur. For. J. 2019, 65, 21–33. [Google Scholar] [CrossRef]
- Leal, S.; Melvin, T.M.; Grabner, M.; Wimmer, R.; Briffa, K.R. Tree-ring growth variability in the Austrian Alps: The influence of site, altitude, tree species and climate. Boreas 2007, 36, 426–440. [Google Scholar] [CrossRef]
- Matisons, R.; Elferts, D.; Krišāns, O.; Schneck, V.; Gärtner, H.; Wojda, T.; Kowalczyk, J.; Jansons, Ā. Nonlinear Weather–Growth Relationships Suggest Disproportional Growth Changes of Norway Spruce in the Eastern Baltic Region. Forests 2021, 12, 661. [Google Scholar] [CrossRef]
- Selås, V.; Piovesan, G.; Adams, J.M.; Bernabei, M. Climatic factors controlling reproduction and growth of Norway spruce in southern Norway. Can. J. For. Res. 2002, 32, 9. [Google Scholar] [CrossRef]
- Hansen, J.; Beck, E. Seasonal changes in the utilization and turnover of assimilation products in 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees 1994, 8, 172–182. [Google Scholar] [CrossRef]
- Holl, W. Seasonal Fluctuation of Reserve Materials in the Trunkwood of Spruce [Picea abies (L.) Karst.]. J. Plant Physiol. 1985, 117, 355–362. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S.G. Growth Control in Woody Plants; Elsevier: Amsterdam, The Netherlands, 1997; ISBN 0080532683. [Google Scholar]
- Gindl, W.; Grabner, M.; Wimmer, R. The influence of temperature on latewood lignin content in treeline Norway spruce compared with maximum density and ring width. Trees 2000, 14, 409–414. [Google Scholar] [CrossRef]
- Hacket-Pain, A.; Ascoli, D.; Berretti, R.; Mencuccini, M.; Motta, R.; Nola, P.; Piussi, P.; Ruffinatto, F.; Vacchiano, G. Temperature and masting control Norway spruce growth, but with high individual tree variability. For. Ecol. Manag. 2019, 438, 142–150. [Google Scholar] [CrossRef]
- Matisons, R.; Elferts, D.; Krišāns, O.; Schneck, V.; Gärtner, H.; Bast, A.; Wojda, T.; Kowalczyk, J.; Jansons, Ā. Non-linear regional weather-growth relationships indicate limited adaptability of the eastern Baltic Scots pine. For. Ecol. Manag. 2021, 479, 118600. [Google Scholar] [CrossRef]
- Akhmetzyanov, L.; Sánchez-Salguero, R.; García-González, I.; Buras, A.; Dominguez-Delmás, M.; Mohren, F.; den Ouden, J.; Sass-Klaassen, U. Towards a new approach for dendroprovenancing pines in the Mediterranean Iberian Peninsula. Dendrochronologia 2020, 60, 125688. [Google Scholar] [CrossRef]
- Fuentes, M.; Salo, R.; Björklund, J.; Seftigen, K.; Zhang, P.; Gunnarson, B.; Aravena, J.-C.; Linderholm, H.W. A 970-year-long summer temperature reconstruction from Rogen, west-central Sweden, based on blue intensity from tree rings. Holocene 2018, 28, 254–266. [Google Scholar] [CrossRef]
- Știrbu, M.-I.; Roibu, C.-C.; Carrer, M.; Mursa, A.; Unterholzner, L.; Prendin, A.L. Contrasting Climate Sensitivity of Pinus cembra Tree-Ring Traits in the Carpathians. Front. Plant Sci. 2022, 13, 855003. [Google Scholar] [CrossRef] [PubMed]
- Walter, H.; Lieth, H. Klimadiagramm-Weltatlas: Von Heinrich Walter Und Helmut Lieth; Gustav Fischer Verlag Jena: Jena, Germany, 1967. [Google Scholar]
- Guijarro, J.A.; Guijarro, M.J.A. Package ‘Climatol’. 2019. Available online: https://cran.r-project.org/web/packages/climatol/climatol.pdf (accessed on 20 April 2020).
- Babst, F.; Frank, D.; Büntgen, U.; Nievergelt, D.; Esper, J. Effect of sample preparation and scanning resolution on the Blue Reflectance of Picea abies. TRACE Proc. 2009, 7, 188–195. [Google Scholar]
- Björklund, J.; Gunnarson, B.E.; Seftigen, K.; Zhang, P.; Linderholm, H.W. Using adjusted Blue Intensity data to attain high-quality summer temperature information: A case study from Central Scandinavia. Holocene 2015, 25, 547–556. [Google Scholar] [CrossRef]
- Wilson, R.; D’Arrigo, R.; Andreu-Hayles, L.; Oelkers, R.; Wiles, G.; Anchukaitis, K.; Davi, N. Experiments based on blue intensity for reconstructing North Pacific temperatures along the Gulf of Alaska. Clim. Past 2017, 13, 1007–1022. [Google Scholar] [CrossRef]
- Wilson, R.; Anchukaitis, K.; Andreu-Hayles, L.; Cook, E.; D’Arrigo, R.; Davi, N.; Haberbauer, L.; Krusic, P.; Luckman, B.; Morimoto, D.; et al. Improved dendroclimatic calibration using blue intensity in the southern Yukon. Holocene 2019, 29, 1817–1830. [Google Scholar] [CrossRef]
- Maxwell, R.S.; Larsson, L.-A. Measuring tree-ring widths using the CooRecorder software application. Dendrochronologia 2021, 67, 125841. [Google Scholar] [CrossRef]
- Bosela, M.; Tumajer, J.; Cienciala, E.; Dobor, L.; Kulla, L.; Marčiš, P.; Popa, I.; Sedmák, R.; Sedmáková, D.; Sitko, R.; et al. Climate warming induced synchronous growth decline in Norway spruce populations across biogeographical gradients since 2000. Sci. Total Environ. 2021, 752, 141794. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Rinn Tech. TSAPWin Scientific: Time Series Analysis and Presentation for Dendrochronology and Related Applications; Rinn Tech: Heidelberg, Germany, 2012. [Google Scholar]
- Holmes, R. Computer assisted quality control. Tree-Ring Bull 1983, 43, 69–78. [Google Scholar]
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree Ring Res. 2001, 57, 205–221. [Google Scholar]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology: Applications in the Environmental Sciences; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1990; ISBN 9401578796. [Google Scholar]
- Schweingruber, F.H. Tree Rings: Basics and Applications of Dendrochronology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1989; ISBN 9400912730. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Jevšenak, J. Daily climate data reveal stronger climate-growth relationships for an extended European tree-ring network. Quat. Sci. Rev. 2019, 221, 105868. [Google Scholar] [CrossRef]
- Beck, W.; Sanders, T.G.M.; Pofahl, U. CLIMTREG: Detecting temporal changes in climate–growth reactions—A computer program using intra-annual daily and yearly moving time intervals of variable width. Dendrochronologia 2013, 31, 232–241. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat. Sci. 1986, 1, 54–75. [Google Scholar] [CrossRef]
- Guiot, J. The bootstrapped response function. Tree-Ring Bull 1991, 51, 39–41. [Google Scholar]
- Biondi, F.; Waikul, K. DENDROCLIM2002: A C++ program for statistical calibration of climate signals in tree-ring chronologies. Comput. Geosci. 2004, 30, 303–311. [Google Scholar] [CrossRef]
- Dixon, P. Bootstrap resampling. In The Encyclopedia of Environmetrics; El-Shaarawi, A.H., Piegorsch, W.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Jevšenak, J.; Levanič, T. dendroTools: R package for studying linear and nonlinear responses between tree-rings and daily environmental data. Dendrochronologia 2018, 48, 32–39. [Google Scholar] [CrossRef] [Green Version]







| Tree Ring Proxy | Series Length | AGR ± SD | Rbar | Mean Sens | Auto Corr. | ||
|---|---|---|---|---|---|---|---|
| Mean | Min | Max | |||||
| Low Elevation Chronologies | |||||||
| TRW | 95 | 79 | 135 | 2.16 ± 0.53 | 0.304 | 0.176 | 0.869 |
| BAI | 1445.7 ± 614.7 | 0.299 | 0.211 | 0.773 | |||
| BI | 2.27 ± 0.17 | 0.146 | 0.063 | 0.548 | |||
| High Elevation Chronologies | |||||||
| TRW | 101 | 68 | 133 | 1.77 ± 0.54 | 0.335 | 0.150 | 0.857 |
| BAI | 1003.6 ± 541.6 | 0.333 | 0.163 | 0.844 | |||
| BI | 2.15 ± 0.20 | 0.287 | 0.077 | 0.459 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, A.; Popa, I.; Roibu, C.-C.; Badea, O.N. Do Different Tree-Ring Proxies Contain Different Temperature Signals? A Case Study of Norway Spruce (Picea abies (L.) Karst) in the Eastern Carpathians. Plants 2022, 11, 2428. https://doi.org/10.3390/plants11182428
Popa A, Popa I, Roibu C-C, Badea ON. Do Different Tree-Ring Proxies Contain Different Temperature Signals? A Case Study of Norway Spruce (Picea abies (L.) Karst) in the Eastern Carpathians. Plants. 2022; 11(18):2428. https://doi.org/10.3390/plants11182428
Chicago/Turabian StylePopa, Andrei, Ionel Popa, Cătălin-Constantin Roibu, and Ovidiu Nicolae Badea. 2022. "Do Different Tree-Ring Proxies Contain Different Temperature Signals? A Case Study of Norway Spruce (Picea abies (L.) Karst) in the Eastern Carpathians" Plants 11, no. 18: 2428. https://doi.org/10.3390/plants11182428