Abstract
Chamaecyparis formosensis is an endemic species of Taiwan, threatened from intensive use and illegal felling. An individual identification system for C. formosensis is required to provide scientific evidence for court use and deter illegal felling. In this study, 36 polymorphic simple sequence repeat markers were developed. By applying up to 28 non-linked of the developed markers, it is calculated that the cumulative random probability of identity (CPI) is as low as 1.652 × 10–12, and the identifiable population size is up to 60 million, which is greater than the known C. formosensis population size in Taiwan. Biogeographical analysis data show that C. formosensis from four geographic areas belong to the same genetic population, which can be further divided into three clusters: SY (Eastern Taiwan), HV and GW (Northwestern Taiwan), and MM (Southwestern Taiwan). The developed system was applied to assess the provenance of samples with 88.44% accuracy rate and therefore can serve as a prescreening tool to reduce the range required for comparison. The system developed in this study is a potential crime-fighting tool against illegal felling.
Similar content being viewed by others
Introduction
Illegal logging is a severe problem in many timber-producing countries. Unplanned felling results in forest degradation, affects forest ecosystems and promotes the spread of pests and pathogens. In addition, large-scale deforestation causes forests to lose their soil and water conservation functions, thus leading to water shortages in the dry season and floods in the rainy season. These disasters brought by illegal or unplanned felling cause significant damages to the country.
Incidents of theft of valuable timber continue to occur, yet the crime investigations are complicated due to the lack of effective measures to present court evidence for conviction. To obtain the molecular evidence linking seized timber to illegally felled stumps, it is necessary to develop an individual identification system for tree species of high economic value, which are often the target of illegal felling.
Chamaecyparis formosensis Masam., also known as False Cypress, is endemic tree species in Taiwan distributed majorly in the cloud forest, a zonal forest type in the mid elevation (1700–2600 m) with extremely high biodiversity1. Gigantic C. formosensis is known for its superb timber quality. Due to its high quality and market value (4050 USD/m3, woodprice.forest.gov.tw), C. formosensis is critically threatened by illegal felling. Unplanned felling and poor management compromised the ecosystem and endangered these endemic species. Similar scenarios also happened to Dalbergia spp. (Leguminosae: Papilionoideae)2,3, Fraxinus excelsior (Oleaceae)4, Swietenia macrophylla (Meliaceae)5, and Intsia palembanica (Fabaceae)6. Moreover, timber production countries suffer from illegal felling, particularly in South-east-Asian, African and South American countries7. Although suspects were arrested on some occasions, lack of direct scientific evidence to link seized timber and stump had led to a failure of conviction in the majority8.
In recent years, countries suffering from serious illegal logging have successively begun to develop DNA-based timber individual identification systems that can provide court evidence for a conviction. The reported technology for individual identification systems includes DNA point difference-based technique SNP (Single Nucleotide Polymorphism)9, and DNA length difference-based techniques SSR (Simple Sequence Repeat)4,6,8,10,11, and INDEL(Insertion/Deletion). SNP, SSR, and INDEL are all co-dominant molecular markers classified into heterozygous and homozygous. Diversified types can be found at the same loci according to the pairwise characteristics of genes in the same allele. For example, human ABO blood type contains three genotypes: IA, IB, and i. When the genotype of the individual is IAIB, IAi, and IBi, it is a heterogeneous combination, showing blood types AB, A, and B respectively. When the genotype of the individual is IAIA, IBIB, ii, it is a homogenous combination, showing blood types A, B, and O, respectively. When reaching enough numbers of the polymorphic molecular markers, they can be used for individual identification and thus can be used to compare the seized timber to the illegally felled stumps. The reported individual identification systems have demonstrated their potentials to provide scientific evidence for court cases4,6,8,9,10,11.
The SSR individual identification technique has been developed for more than 30 years and has been widely used in DNA paternity testing, forensic examination, victim identification, and animal individual identification12,13. SSR marker is a co-dominant and highly reproducible DNA marker with the following characteristics: it has a high degree of polymorphism; it is abundant and evenly distributed in eukaryotic genome; most of them are not functional and can be efficiently and economically tested by PCR (polymerase chain reaction); last, the length is generally short which provides a higher opportunity to be amplified when applied to the lysed sample13,14. Therefore, SSR is the most commonly used method for individual identification systems15,16. To protect the cypress resources in Taiwan, we have developed and adapted several polymorphic SSR markers17 for individual identification.
In the illegal felling crime case reports C. taiwanensis8 and F. excelsior4, it is demonstrated that the SSR individual identification system can provide scientific evidence that is considered acceptable by court. In these cases, the individual identification system developed with genetic markers for those species were used to link seized timbers and victim trees, while considering the random probability of the same genotype appearing in the population. Therefore, convincible scientific proof with a confidence level close to 100% was accepted as court evidence for crime conviction.
The legality of wood products usually depends on their source18. SSR is also often used in genetic diversity and population structure analysis of species14,19, and can further predict species' geographic provenance and distribution. Genetic methods have been applied to confirm the source and trade routes of protected species20,21. However, filing the DNA of every individual is not feasible even if C. formosensis has been listed as an endangered species. Therefore, it is important to analyze the genetic variation and population structure of C. formosensis. Genotyping can reveal the provenance of the timber and greatly reduce the range of possible plant sources.
Due to the extensive planned logging and rampant illegal logging in the last century, C. formosensis has been listed as an endangered species by the IUCN Red Book (International Union for conservation of nature red list of threatened species). It has become one of the most concerning issues whether C. formosensis has lost its genetic diversity due to excessive logging. From conservation perspective, SSR marker can be used to find high population genetic diversity areas, to understand the local species’ genetic structure composition of these areas, and to further provide necessary conservation measures and management to these areas. SSR marker is an effective tool for studying the diversity of populations and providing harmonised standards (e.g. the number of allelic (A), observed heterozygosity (Ho), expected heterozygosity (He), the inbreeding coefficient (Fis), the fixation index (Fst)) for comparison with other species22.
The primary purpose of this study is to develop C. formosensis SSR individual identification system, which provides high discrimination power against genetic variation, in order to prevent the occurrence of illegal felling. Moreover, the biogeographical analysis of C. formosensis also facilitates providing provenance information of the seized timber. In addition to being a long-developed individual identification tool15,16, SSR is also often used in plant conservation and breeding23,24. The SSR markers developed in this study can also support the future C. formosensis afforestation by selecting mother trees with higher diversity.
Result and discussion
Development of new SSR markers for C. formosensis
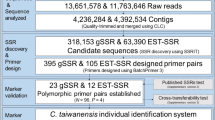
For a higher accuracy of court's judgment on illegal felling, it is necessary to establish a complete forensic system. Although some SSR markers of cypress have been published17,25,26,27, the reported detection rates for dried timber were only 20–40%8,9. Therefore, it is necessary to develop more SSR markers as a contingency plan. When the sample is in a poor condition, more markers can be applied in order to achieve the threshold of combined power of discrimination (CPD) required for successful comparison between seized timbers and victim trees. In order to maximize potential loci, Next generation sequence (NGS) methods were used. In this study 3 DNA library were constructed. We used the Illumina MiSeq platform (2 × 301 bp; Illumina, San Diego, California, USA) to sequence the DNA libraries (Fig. 1. and Supplementary 1.).
A total of 70,325,072 raw reads were produced. The raw reads were deposited in the NCBI BioProject (PRJNA454510). After quality-trimming to the raw reads with CLC Genomics Workbench version 10 (QIAGENE, Aarhus, Denmark), 70,319,509 contigs were generated with the length between 133 and 146 bp on average. De novo assembly was conducted with the following parameters: contig number 208,467, minimum length of contigs 18 bp, maximum length contigs 108,928 bp, and average length contigs 491 bp. The sequence was assembled with software CLC Genomics Workbench version 10, and the length of the assembly sequence was 102,281,642 bp.
A sum of 78,250 SSR containing sequences was screened by MISA (v 1.0, MicroSAtellite)28. We newly designed 100 candidate SSR primer pairs for testing in C. formosensis by BatchPrimer329.
There are 9 validated SSR markers that are polymorphic (success rate 9.00%) were registered in GenBank in NCBI (Table 1) and passed for cross-species tests (Supplementary 2 and 3).
Unlike the traditional SSR cloning method, with next-generation sequencing technology, it is easy to obtain a significant amount of SSR containing sequences from sequenced genomes30. However, transforming candidate SSR primer pairs into validated SSR markers is still a time-consuming and expensive step. Qualified SSR markers need to succeed in PCR amplification, have good peak pattern quality with minor stuttering, and be free of non-amplifying (invalid) alleles. The turnover rate from candidate SSR primer pairs to validated SSR markers varies from species to species30,31. The success rate in Chamaecyparis plants is between 5.24% and 9.27%8,17,25,26,32.
Developing C. formosensis individual identification system
In this study, newly developed 9 validated SSR markers and other 27 validated SSR markers17 polymorphic SSR markers were analyzed against 92 individuals from 4 geographic areas (MM, HV, GW, SY, Fig. 2 and Supplementary 1). The results of developed 36 SSR markers are summarized in Table 2. Among the 92 individuals in this study, each number of alleles of SSR is between 2 and 27, with an average of 7.916. The levels of observed heterozygosity (Ho)33 are from 0.000 to 0.891, with an average of 0.414. The levels of expected heterozygosity (He)33 range from 0.103 to 0.906, with an average of 0.565. Significant (P < 0.001) deviations of Hardy–Weinberg equilibrium (HWE)34 were detected in 23 SSR loci: Cred47, 225, 231, 236, 242, 248, 249, 250, 253, 260, 262, 276, 277, 280, 603, 610, 628, 640, 641, 674, 678, 682, 683. Ho is the actual proportion of heterozygous individuals in each locus within the population, whereas the He is the expected value estimated per HWE. Ho and He are among the most widely used parameters in estimating genetic diversity in a population. The population structural and even historical information can be obtained from Ho and He. When Ho = He, it means that the population is random mating. When Ho < He, it means that the population is inbreeding. When Ho > He, it means that the population is outcrossing33. Most of these loci (36 tested) are Ho < He (except Cred211, Cred220, Cred225, Cred248, Cred276, Cred281, Cred297, Cred298), suggesting the population of C. formosensis has a low genetic divergence and is an inbred strain. HWE describes that under ideal conditions, there exists no mutations, no natural selection, no individuals moving in or out, the population is infinitely large, and random mating within the population. Therefore, gene frequency does not change over time or generation. However, there will always be one or more interfering factors (e.g. genetic drift, natural selection, mutation, gene flow, population bottleneck, founder effect, and inbreeding.) affecting gene frequency in nature35. Therefore, HWE is difficult to achieve in nature. In this study, 23 loci out of 36 markers deviated from HWE (63.89% deviation rate). The reason for this deviation could be artificial selection, non-panmixia or genetic drift.
The biogeographic information of Chamacyparis formosensis in this study. A total of 92 samples composed of 20 MM, 25 HV, 23 GW, and 24 SY individuals were analyzed (a) Biogeographic analysis data suggests that the samples fall into three genetical categories: SY (Eastern Taiwan), HV & GW (Northwestern Taiwan), and MM (Southwestern Taiwan) (b)–(e) The red spots represent the individuals that have been mis-assigned (denoted as M in figure legend) from provenance simulation result. N: the number of individuals, M: the number of mis-assigned.
Polymorphism information content, or power of information content (PIC), is an index of the relative ability of the SSR marker's genetic variability. The higher the polymorphism of marker's genotype, the higher the PIC value36. Polymorphic markers were highly informative (PIC > 0.50), reasonably informative (0.50 > PIC > 0.25), and slightly informative (PIC < 0.25). Power of discrimination (PD)37 refers to the ability of genetic markers to distinguish individuals within a population. Obviously, in a population with more allele types and evenly distributed genotypes, the low probability of two random individuals having the same genotype, and the system can identify the greater probability of two random individuals. Probability of identity (PI)38 is the probability of two individuals with the same genotype. PD = 1-PI . The value of PIC, PD and PI of individual markers reflects its identification ability in the individual identification system. The greater PIC and PD, the lower PI in value, suggesting the higher identification ability of the marker, and vice versa. The levels of PIC range from 0.097 to 0.876, with an average 0.528. The levels of PD range from 0.102 to 0.885, with an average 0.567. The levels of PI range from 0.114 to 0.897, with an average 0.431. There were 19 out of 36 markers with PIC greater than 0.5, and the mean of these 36 markers PIC values was greater than 0.5, suggesting the markers have a high identification ability. The results of PD and PI correspond to those of PIC. The highly informative markers presented in PIC also show higher identification ability in PD and PI.
Significant linkages (P < 0.001) were detected among Cred35/229/277 (Group 1), Cred47/298 (Group 2), Cred231/249/253/262 (Group 3), Cred281/297 (Group 4), Cred603/683 (Group 5) and Cred640/678/682 (Group 6) with GENEPOP 4.239, suggesting the abovementioned group located in the same linkage group (Table 2). When identifying several independent polymorphic genetic markers simultaneously (polymorphic markers located in different linkage groups), the combined probability of identity (CPI) is the product of the PI of each genetic marker. At this time, CPI will be greatly reduced, and the combined power of discrimination (CPD) will become very high. As defined above, CPI + CPD = 1. The credibility of the individual identification system is calculated based on "Random match probability in population size and confidence levels’" published by Budowle et al.40. Confidence levels (CL) = (1 – CPI)N, where N is number of individuals.
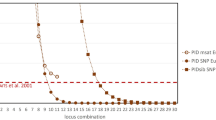
The individual identification system was applied to illegal felling cases8. When the seized timber and the victim tree are identified as the same particular plant, under the considerations of fairness and objective, the court usually adopts 99.99%, 99% or 95% confidence level as the credibility standard41, ISO ISO/IEC 17,025). In this study, the locus with the lowest PI within a linkage group was used to calculate the CPI (Table 3). The CPI decreased along with accumulation of loci and the PI of each locus were sorted in ascending order. The system can accumulate up to 28 loci without linkage. When reaching its maximum capability, even under most strict standard (confidence level 99.99%) dictated by court, this system can be used to identify 60 million C. formosensis, with CPI as low as 1.652 × 10–12, CPD as high as 0.999999999998348 (almost equal to 1), beyond the known population size of 32.06 ± 3.20 million C. formosensis42. Under ideal conditions, a minimum of 6 loci can be applied to the system, with an identifiable C. formosensis population of 2,900 under 95% confidence level. The CPI is as low as 1.728 × 10–5, and CPD is as high as 0.999982712603209 (Table 3).
One of the problems with SSR marker is the appearance of null alleles. One possible cause of SSR null alleles is poor primer annealing caused by the nucleotide sequence divergence of the flanking primer on one or both sides (for example, point mutation or indel in the primer sequence)43. In addition, due to the competitive nature of PCR, smaller alleles usually have a higher amplification efficiency than larger alleles. Therefore, only the smaller of the two alleles can be detected from heterozygous individuals. The null alleles caused by differential amplification can usually be seen by loading more samples or adjusting the contrast44. The third cause of null alleles may be due to inconsistent quality or the low quantity of DNA templates. Some loci are relatively easy to amplify, yet others cannot be amplified within the same DNA preparation45. When a null allele is present, the observed genotype represents one of the several possible true genotypes46. SSR markers inevitably produce null alleles, and each SSR marker has a different background for null alleles.
Dakin et al. (2004)44 reviewed 233 publications by examining how authors detect and deal with null alleles and the methods used to estimate the frequency of null alleles across articles. The authors demonstrate that the frequency of simulated null alleles is usually overestimated, which will lead to underestimating the usability of this marker. It was misunderstood that the existence of null alleles will reduce the availability of paternity testing, individual identification, and population genetic research. However, it has been demonstrated that null alleles do not change the overall result on assignment testing43,44. Compared with the presence of null alleles, increasing the number of loci and the degree of genetic differentiation has a more significant impact on the accuracy of assignment testing. This argument is valuable for studying SSR markers and populations prone to invalid alleles, as it allows researchers to use loci affected by invalid alleles43,44.
In Huang et al.8 where C. taiwanensis individual identification system was applied to an illegal felling conviction case, CPD calculations exclude any markers that show homozygous PCR results per ISO/IEC 17,025. The CPDs are calculated only from the possibilities of the markers found in timber and tree samples simultaneously. Null alleles and PCR fail will only reduce the identification rate but will not cause seized timber and victim tree from different individuals to be identified as the same source. However, this is not to say that efforts should not be made to use loci that display low-frequency null alleles. On the contrary, markers that are less prone to invalid alleles should always be preferred because they are less ambiguous and are more potent in assignment testing. However, before many individual identification markers are developed and optimised, the impact of null alleles should not be overemphasized, as it reduces the usability of markers43,44.
Population genetics analysis
Fis, by definition Fis = 1-Ho/He, is the inbreeding coefficient of an individual concerning the local subpopulation. When Ho < He, then Fis > 0, indicating that the population is an inbreeding. The 36 polymorphic SSRs were used to evaluate the genetic diversity parameters of the four groups (MM, HV, GW, SY) (Table 4). The number of alleles (A) for each locus is 4.417 and 5.444. Ho and He are ranged from 0.376 to 0.506 and from 0.474 to 0.583, respectively. All the groups show positive inbreeding coefficients, suggesting these four groups are inbreeding lines.
The fixation index (Fst) estimates population differentiation due to genetic structure47. A higher Fst value means a higher degree of difference between populations. When Fst is less than 0.05, there is no differentiation among populations. When Fst is between 0.05 and 0.15, there is low differentiation among populations. On the other hand, the estimation of the number of migrants (Nm) is gene flow value47. If Nm is more than one, genes frequently exchange, which counteracts the genetic drift and prevents the population differentiation48. If Nm is greater than four, the population is a random mating49. The analyses of Fst and Nm of the four geographic areas were conducted by GeneAlex 6.50350 (Table 5). The Fst value between HV and GW was 0.035, suggesting no population differentiation in these two populations. The highest Fst value (0.074) was found between HV and MM. The Fst values ranged from 0.056 to 0.065 were found between the rest geographic areas, indicating a low differentiation in these geographic areas. The highest Nm value (6.832) was found between HV and GW, whereas the lowest value (3.141) was between HV and MM. The Nm values of four geographic areas were greater than 1 (between 3.141 and 6.832), suggesting a frequent gene exchange between the four geographic regions, which offsets genetic drift and prevents population differentiation. For GW/MM (Nm = 4.022), GW/HV (Nm = 6.382), the Nm values of the population are more significant than four, suggesting that these populations are random mating.
STRUCTURE analysis51,52 was used to analyze the population genetic structure of C. formosensis (Fig. 3), and the Delta K value was calculated to obtain the optimal number of clusters. K and Delta K are shown in Fig. 3a. The individuals of C. formosensis were most likely to be three clusters (Fig. 3b): the SY located in Eastern Taiwan is an independent cluster, the MM located in Southwestern Taiwan is another cluster, whereas the two HV and GW geographic areas are in the same genetic cluster. The results of Fst (Table 5), Nm (Table 5) and STRUCTURE analyses (Fig. 3) show that C. formosensis of the four geographical areas belongs to the same genetic population. The Fst and STRUCTURE analyses suggest that the samples fall into three clusters. The hypothesis that Taiwan Island is one of the plant refuges during the Quaternary glaciation53,54 may help to explain the results. The study of historical biogeography and phylogeny of cypress54 suggested that C. formosensis in Taiwan diverged from Chamaecyparis in Japan 2.9 million years ago. The arrival of the Quaternary glaciation led to species extinction and the continued retreat of species to lower latitudes55; thus Taiwan Island became a refuge for many ancient species, such as Juniperus morrisonicola (Cupressaceae)56, Abies kawakamii (Pinaceae)57, Castanopsis carlesii (Fagaceae)58. After the glaciation, species spread from the refuge to the surrounding areas and created species diversity across the latitude gradient59. In our results, the low polymorphism of C. formosensis probably indicates that they originally derived from the same large population during the glaciation. After the glacial retreat, these four C. formosensis clusters spread out from the refuge and formed the four populations due to geographic isolation.
Studies55,60 also show that, based on molecular evidence, many plants (eg. Cunninghamia konishii, Cyclobalanopsis glauca, Trochodendron aralioides) in Taiwan island have high genetic diversity, higher than that of mainland China and Japanese archipelago. This remarkable high genetic diversity is associated with the Ice Age history in Taiwan55,60. The low genetic diversity of C. formosensis differs from most Taiwanese plants but is similar to another endangered plant of the genus Cypress, C. taiwanensis, in Taiwan Island (A = 6.507, Ho = 0.392, He = 0.501)8. Compared to C. obtusa, an endangered cypress plant in the Japanese archipelago, C. formosensis is also inbreeding (Fis = 0.034), but the degree of genetic diversity (A = 23.9) is significantly lower. One possible explanation of the low genetic diversity is that a large population of C. formosensis was divided into several smaller populations after ancient glacial retreat in Taiwan, and then they were recently overexploited by humans (REF).
GENECLASS v. 2.061 was applied to analyse the provenance of 92 individuals independently. The probability of samples returning to the correct provenance is 95.00% (MM), 88.00% (HV), 69.57% (GW), and 100.00% (SY), with an overall mean correct rate of 88.04% (Table 6). Three HV individuals were misassigned to GW and four GW individuals were misassigned to HV, corresponding to the observation that HV and GW are the same clusters. However, three GW individuals were misassigned to MM, possibly because the geographic location of GW is between HV and MM. Therefore, GW has characteristics of north and south at the same time. Likelihood, one MM was mis-assigned to GW, further supporting the inference that there is partial gene exchange between MM and GW. Our data show that the populations in eastern (SY) and western Taiwan (the rest populations) have distinct genotype differences. Within the western populations, the northern (HV) and the southern ones (MM) have obvious differences. Therefore, when seizing timbers in the future, the genotype can be served as a prefilter to infer the geographic area of the victim tree if the provenance is found to be MM, HV or SY. A further inspection is required if the provenance is GW because of the existence of gene exchange between nearby geographic areas.
Conclusions
In this study, a C. formosensis individual identification system was built with 36 polymorphic SSR markers. When 28 non-linked SSR markers are applied, the system is capable of identifying 60 million C. formosensis individuals with a confidence level of 99.99%. The lowest CPI is 1.652 × 10–12, and the highest CPD is 0.999999999998348. This system can provide the scientific evidence to link seized timbers and victim trees required the illegal felling court cases and facilitate future legal sales by profiling timbers. Through population genetics analysis, the system can provide provenance information, which would significantly enhance the efficiency by reducing the range required for investigation. The polymorphic markers developed in this study can be further applied to the conservation and breeding of the endangered species C. formosensis.
Materials and methods
Development of new SSR markers for C. formosensis
In order to develop SSR markers for individual identification, we constructed three DNA libraries. Three C. formosensis individuals from QL (Voucher no. Chung 4450) and SY (Voucher no. Chung 4905, 4906) were used for DNA library preparation. To build three DNA libraries, genomic DNA was extracted from fresh leaves using the VIOGENE plant DNA extraction kit (VIOGENE, New Taipei City, Taiwan). The DNA libraries were sequenced using the Illumina MiSeq System (2 × 301 bp paired-end; Illumin, San Diego, California, USA) at Tri-I Biotech (New Taipei City, Taiwan).
Bioinformatics analysis was conducted with CLC Genomics Workbench version 10 (QIAGENE, Aarhus, Denmark). The raw reads were prescreened to remove adapter sequences and reads with greater than 0.01 error or an average quality less than QV20. The trimmed sequences were further subjected to de novo assembly.
MISA (MIcroSAtellite v 1.0)28 was applied to screen the SSR containing sequences from contigs. To design SSR primers, sequences with at least five di-, tri-, tetra-, penta-, and hexa-nucleotide repeats were selected using BatchPrimer329, with optimized conditions set length at 18–23 bp, melting temperature 45–62 °C, and product size of 80–300 bp.
A total of 100 candidate SSR primer pairs were newly designed in this study. These markers were subjected to validation test on 92 samples from four C. formosenses geographic areas (MM, HV, GW, SY see Supplementary 1). The samples DNA used in marker validation were extracted using the VIOGENE plant DNA extraction kit (VIOGENE, New Taipei City, Taiwan). The PCR reaction was conducted with a final volume 20 μL containing 2 ng of genomic DNA, 0.25 μL of 10 μM each primer and 10 μL of Q-Amp 2 × Screening Fire Taq Master Mix (Bio-Genesis Technologies, Taipei, Taiwan). The following PCR process was conducted: an initial denaturation of 95 °C for 2 min; 30 cycles of 95 °C for 45 s, a primer-specific annealing temperature for 45 s, and 72 °C for 45 s; followed by a 15-min extension at 72 °C (Table 1). The amplified products were evaluated on the ABI 3500 (Applied Biosystems, Waltham, Massachusetts, USA) with GeneScan 600 LIZ Size Standard (Applied Biosystems). Fragment size was determined by using GeneMapper ID-X v1.6 (Applied Biosystems). The capillary electrophoresis diagrams for genotyping are shown in Supplementary 4.
The cross-species transferability of the designed markers was tested in Chamaecyparis taiwanensis; for details, see Supplementary 2 and 3.
Developing C. formosensis individual identification system
Marker analysis was conducted by combining 27 pairs published SSR markers17 with 9 validated SSR markers abovementioned. GenAlex 6.51b250 was used to calculate number of alleles (A), observed heterozygosity (Ho), expected heterozygosity (He), Hardy–Weinberg equilibrium (HWE). PowerMarker V3.2562 was used to calculate polymorphism information content or power of information content (PIC)63. Power of discrimination (PD)37, PD = 1 − ΣPi2, where Pi is the frequency of genotype i . Probability of identity (PI)38, PI = 1 − PD. The combined power of discrimination (CPD)37, here we calculated CPD of 28 markers. CPD = 1 – [(1 – PD1)(1 – PD2)…(1 – PD28)].The combined probability of identity (CPI)38. Microsoft Excel (Microsoft Office 2016) was used to calculate PD, PI, CPD, CPI. GENEPOP 4.239 was used to test for linkage disequilibrium.
Population genetics analysis
Genetic diversity parameters, genetic differentiation and gene flow among 4 geographic areas (MM, HV, GW, SY) were analyzed using Fis, Fst and Nm by GenAlex 6.50350.
The population genetic structure was analyzed using STRUCTURE 2.3.452. The program was run for K = 1 to 5 clusters with 20 independent runs to assess simulation stability. Each simulation was run for an initial 1,000,000 burn-in period followed by 100,000 replications based on the Markov chain Monte Carlo (MCMC)64. The best grouping was evaluated by Delta K64 in Structure Harvester Web v0.6.9465. Bar graphs were generated by CLUMPP 1.1.266 for K ideal.
Individual provenance simulation was conducted with GENECLASS v. 2.061 on every 92 individuals independently. A pairwise simulation was also conducted on the pooled database deducted the sample itself.
Plant collecting permit declaration
With legislation compliance of experimental materials, we hereby declare that all of our experimental research and field studies on plants, either cultivated or wild, including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation.
Software and data use declaration
In this research, the software and the data generated by the software (including commercial software, open-licensed software) are used legally in accordance with regulations, which allow to reproduction, distribution, transmit and modification works (including commercial use).
Data availability
Raw sequence information and developed SSR primer pairs have been deposited to NCBI (BioProject ID PRJNA454510); GenBank accession numbers are provided in Table 1.
References
Hwang, S. Y., Lin, H. W., Kuo, Y. S. & Lin, T. P. RAPD variation in relation to population differentiation of Chamaecyparis formosensis and Chamaecyparis taiwanensis. Bot. Bull. Acad. Sinica 42, 173–179 (2001).
Kite, G. C. et al. Dalnigrin, a neoflavonoid marker for the identification of Brazilian rosewood (Dalbergia nigra) in CITES enforcement. Phytochemistry 71, 1122–1131. https://doi.org/10.1016/j.phytochem.2010.04.011 (2010).
Espinoza, E. O., Wiemann, M. C., Barajas-Morales, J., Chavarria, G. D. & McClure, P. J. Forensic analysis of CITES-protected Dalbergia timber from the Americas. IAWA J. 36, 311–325 (2015).
Tereba, A., Woodward, S., Konecka, A., Borys, M. & Nowakowska, J. A. Analysis of DNA profiles of ash (Fraxinus excelsior L) to provide evidence of illegal logging. Wood Sci. Technol. 51, 1377–1387. https://doi.org/10.1007/s00226-017-0942-5 (2017).
Cabral, E. C. et al. Wood typification by Venturi easy ambient sonic spray ionization mass spectrometry: the case of the endangered Mahogany tree. J. Mass Spectrom. 47, 1–6. https://doi.org/10.1002/jms.2016 (2012).
Lowe, A. J., Wong, K. N., Tiong, Y. S., Iyerh, S. & Chew, F. T. A DNA Method to verify the integrity of timber supply chains; confirming the legal sourcing of merbau timber from logging concession to sawmill. Silvae Genetica 59, 263–268. https://doi.org/10.1515/sg-2010-0037 (2010).
Dormontt, E. E. et al. Forensic timber identification: It’s time to integrate disciplines to combat illegal logging. Biol. Cons. 191, 790–798. https://doi.org/10.1016/j.biocon.2015.06.038 (2015).
Huang, C.-J. et al. Development and technical application of SSR-based individual identification system for Chamaecyparis taiwanensis against illegal logging convictions. Sci. Rep. 10, 1–14 (2020).
Dormontt, E. et al. Forensic validation of a SNP and INDEL panel for individualisation of timber from bigleaf maple (Acer macrophyllum Pursch). Foren. Sci. Int. Genet. 46, 102252 (2020).
Hung, K.-H., Lin, C.-H. & Ju, L.-P. Tracking the geographical origin of timber by DNA fingerprinting: a study of the endangered species Cinnamomum kanehirae in Taiwan. Holzforschung 71, 853–862 (2017).
Jolivet, C. & Degen, B. Use of DNA fingerprints to control the origin of sapelli timber (Entandrophragma cylindricum) at the forest concession level in Cameroon. Foren. Sci. Int. Genet. 6, 487–493. https://doi.org/10.1016/j.fsigen.2011.11.002 (2012).
Jeffreys, A. J., Wilson, V. & Thein, S. L. Individual-specific ‘fingerprints’ of human DNA. Nature 316, 76–79 (1985).
Robinson, A. J., Love, C. G., Batley, J., Barker, G. & Edwards, D. Simple sequence repeat marker loci discovery using SSR primer. Bioinformatics 20, 1475–1476 (2004).
Ali, A. et al. Genetic diversity and population structure analysis of Saccharum and Erianthus genera using microsatellite (SSR) markers. Sci Rep 9, 395. https://doi.org/10.1038/s41598-018-36630-7 (2019).
Jobling, M. A. & Gill, P. Encoded evidence: DNA in forensic analysis. Nat Rev Genet 5, 739–751. https://doi.org/10.1038/nrg1455 (2004).
Butler, J. M. Genetics and genomics of core short tandem repeat loci used in human identity testing. J. Forensic Sci. 51, 253–265 (2006).
Huang, C. J. et al. Isolation and characterization of SSR and EST-SSR loci in Chamaecyparis formosensis (Cupressaceae). Appl. Plant Sci. 6, e01175. https://doi.org/10.1002/aps3.1175 (2018).
Finch, K. N. et al. Predicting the geographic origin of Spanish Cedar (Cedrela odorata L.) based on DNA variation. Conserv. Genet. 21, 625–639 (2020).
Dorji, J., Tamang, S., Tshewang, T., Dorji, T. & Dorji, T. Y. Genetic diversity and population structure of three traditional horse breeds of Bhutan based on 29 DNA microsatellite markers. PLoS ONE 13, e0199376 (2018).
Paredes-Villanueva, K. et al. Nuclear and plastid SNP markers for tracing Cedrela timber in the tropics. Conserv. Genet. Resour., 1–6 (2019).
Blanc-Jolivet, C., Yanbaev, Y., Kersten, B. & Degen, B. A set of SNP markers for timber tracking of Larix spp. in Europe and Russia. Fores. Int. J. Forest Res. 91, 614–628 (2018).
Morgante, M. & Olivieri, A. PCR-amplified microsatellites as markers in plant genetics. Plant J. 3, 175–182 (1993).
implications for conservation and breeding. Penha, J. et al. Estimation of natural outcrossing rate and genetic diversity in Lima bean (Phaseolus lunatus L. var. lunatus) from Brazil using SSR markers. Genet. Resour. Crop Evol. 64, 1355–1364 (2017).
Yang, H., Zhang, R., Jin, G., Feng, Z. & Zhou, Z. Assessing the genetic diversity and genealogical reconstruction of cypress (Cupressus funebris Endl.) breeding parents using SSR markers. Forests 7, 160 (2016).
Matsumoto, A. et al. Development and polymorphisms of microsatellite markers for hinoki (Chamaecyparis obtusa). Mol. Ecol. Notes 6, 310–312. https://doi.org/10.1111/j.1471-8286.2006.01212.x (2006).
Nakao, Y., Iwata, H., Matsumoto, A., Tsumura, Y. & Tomaru, N. Highly polymorphic microsatellite markers in Chamaecyparis obtusa. Can. J. For. Res. 31, 2248–2251. https://doi.org/10.1139/cjfr-31-12-2248 (2001).
Kim, Y. M., Shin, Y. S. & Jeong, J. H. Development and characterization of microsatellite primers for Chamaecyparis obtusa (Cupressaceae). Applications in plant sciences 4, 1500136 (2016).
Thiel, T., Michalek, W., Varshney, R. & Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theoretical and applied genetics 106, 411–422 (2003).
You, F. M. et al. BatchPrimer3: a high throughput web application for PCR and sequencing primer design. BMC Bioinformatics 9, 253. https://doi.org/10.1186/1471-2105-9-253 (2008).
Zalapa, J. E. et al. Using next-generation sequencing approaches to isolate simple sequence repeat (SSR) loci in the plant sciences. Am. J. Bot. 99, 193–208 (2012).
Gardner, M. G., Fitch, A. J., Bertozzi, T. & Lowe, A. J. Rise of the machines–recommendations for ecologists when using next generation sequencing for microsatellite development. Mol. Ecol. Resour. 11, 1093–1101 (2011).
Iwaizumi, M., Watanabe, A. & Isoda, K. Primer note: Development of highly polymorphic nuclear microsatellite markers for Hinoki (Chamaecyparis obtusa). Silvae Genetica 60, 62–65 (2011).
Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. 70, 3321–3323 (1973).
Rodriguez, S., Gaunt, T. R. & Day, I. N. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am. J. Epidemiol. 169, 505–514 (2009).
Masel, J. Rethinking Hardy-Weinberg and genetic drift in undergraduate biology. BioEssays 34, 701–710 (2012).
Pan, Y.-B. Highly polymorphic microsatellite DNA markers for sugarcane germplasm evaluation and variety identity testing. Sugar Tech. 8, 246–256 (2006).
Fisher, R. Standard calculations for evaluating a blood-group system. Heredity 5, 95 (1951).
Jones, D. A. Blood Samples : Probability of Discrimination. J. Foren. Sci. Soc. 12, 355–359. https://doi.org/10.1016/s0015-7368(72)70695-7 (1972).
Raymond, M. & Rousset, F. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J. Heredity 86, 248–249 (1995).
Budowle, B., Chakraborty, R., Carmody, G. & Monson, K. L. Source attribution of a forensic DNA profile. Foren. Sci. Commun. 2, 6 (2000).
Wall, W. Genetics & DNA technology: legal aspects. (Routledge-Cavendish, 2002).
Qiu, L. W., Huang, Q. X., Wu, C. C. & Hsieh, H. T. (Taipei, 2015).
Carlsson, J. Effects of microsatellite null alleles on assignment testing. J. Hered. 99, 616–623 (2008).
Dakin, E. & Avise, J. Microsatellite null alleles in parentage analysis. Heredity 93, 504–509 (2004).
Gagneux, P., Boesch, C. & Woodruff, D. Microsatellite scoring errors associated with noninvasive genotyping based on nuclear DNA amplified from shed hair. Mol. Ecol. 6, 861–868 (1997).
Wagner, A., Creel, S. & Kalinowski, S. Estimating relatedness and relationships using microsatellite loci with null alleles. Heredity 97, 336–345 (2006).
Wright, S. Isolation by distance. Genetics 28, 114 (1943).
Wright, S. Evolution and the genetics of populations: Vol. 2. The theory of gene frequencies. (1969).
Hartl, D. & Clark, A. Principles of population genetics (Sinauer Assoc. Inc, 1989).
Peakall, R. & Smouse, P. E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 28, 2537–2539 (2012).
Falush, D., Stephens, M. & Pritchard, J. K. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164, 1567–1587 (2003).
Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 155, 945–959 (2000).
Qian, H. & Ricklefs, R. E. Large-scale processes and the Asian bias in species diversity of temperate plants. Nature 407, 180–182 (2000).
Wang, W. P., Hwang, C. Y., Lin, T. P. & Hwang, S. Y. Historical biogeography and phylogenetic relationships of the genus Chamaecyparis (Cupressaceae) inferred from chloroplast DNA polymorphism. Plant Syst. Evol. 241, 13–28. https://doi.org/10.1007/s00606-003-0031-0 (2003).
Chiang, T.-Y. & Schaal, B. A. Phylogeography of plants in Taiwan and the Ryukyu Archipelago. Taxon 55, 31–41 (2006).
Huang, C.-C. et al. Multilocus analyses reveal postglacial demographic shrinkage of Juniperus morrisonicola (Cupressaceae), a dominant alpine species in Taiwan. PLoS ONE 11, e0161713 (2016).
Shih, F. L., Hwang, S. Y., Cheng, Y. P., Lee, P. F. & Lin, T. P. Uniform genetic diversity, low differentiation, and neutral evolution characterize contemporary refuge populations of Taiwan fir (Abies kawakamii, Pinaceae). Am. J. Bot. 94, 194–202 (2007).
Cheng, Y. P., Hwang, S. Y. & Lin, T. P. Potential refugia in Taiwan revealed by the phylogeographical study of Castanopsis carlesii Hayata (Fagaceae). Mol Ecol 14, 2075–2085 (2005).
Qian, H. A comparison of the taxonomic richness of temperate plants in East Asia and North America. Am. J. Bot. 89, 1818–1825 (2002).
Chiang, Y. C. et al. Contrasting phylogeographical patterns between mainland and island taxa of the Pinus luchuensis complex. Mol. Ecol. 15, 765–779 (2006).
Piry, S. et al. GENECLASS2: a software for genetic assignment and first-generation migrant detection. J. Hered. 95, 536–539 (2004).
Liu, K. & Muse, S. V. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21, 2128–2129 (2005).
Botstein, D., White, R. L., Skolnick, M. & Davis, R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 32, 314 (1980).
Evanno, G., Regnaut, S. & Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 14, 2611–2620. https://doi.org/10.1111/j.1365-294X.2005.02553.x (2005).
Earl, D. A. & vonHoldt, B. M. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 4, 359–361, https://doi.org/10.1007/s12686-011-9548-7 (2011).
Jakobsson, M. & Rosenberg, N. A. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23, 1801–1806. https://doi.org/10.1093/bioinformatics/btm233 (2007).
Brown, A. H. & Weir, B. S. Measuring genetic variability in plant populations. Isozym. Plant Genet. Breed. Part A, 219–239 (1983).
Acknowledgements
The authors would like to thank Dr. Kuo-Fang Chung (Biodiversity Research Center, Academia Sinica, Taiwan) for his support in research funding and facilities. The authors would like to thank Dr. Chaolun Allen Chen and Dr. Shu-Miaw Chaw (Biodiversity Research Center, Academia Sinica, Taiwan) for their support in research facilities. This work was financially supported by Ministry of Science and Technology, Taiwan (grant no. MOST 104-2321-B-002-056) and Ministry of Justice, Taiwan (Grant no. 109-1301-05-17-02).
Author information
Authors and Affiliations
Contributions
C.J.H. conceived, designed and conducted the experiments, wrote the main manuscript text, drew the figures and tables, collected the samples, secured the funding, and submitted the manuscript. F.H.C. edited the manuscript. Y.S.H. edited the manuscript and assisted in drawing the figures and tables. Y.C.T. and Y.M.H. performed the experiments. Y.H.T. edited the manuscript. C.E.P. performed the data analysis. C.T.H. drew the figures and collected the samples. C.H.C. edited the manuscript. Y.S.C. performed the data analysis. S.C.L., Y.T.Y., S.Y.H., and H.C.H. collected the samples and performed the experiments. C.T.W. collected samples. C.T.C. secured the funding. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, CJ., Chu, FH., Huang, YS. et al. SSR individual identification system construction and population genetics analysis for Chamaecyparis formosensis. Sci Rep 12, 4126 (2022). https://doi.org/10.1038/s41598-022-07870-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07870-5
This article is cited by
-
A taxonomic revision of the genus Angelica (Apiaceae) in Taiwan with a new species A. aliensis
Botanical Studies (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.