Integrated Pest Management for Christmas Tree Production: A ...
Integrated Pest Management for Christmas Tree Production: A ...
Integrated Pest Management for Christmas Tree Production: A ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
IN MEMORIAM<br />
We dedicate this publication to Dr. Paul Heller and<br />
Dr. William Merrill <strong>for</strong> their contribution to the<br />
<strong>Christmas</strong> <strong>Tree</strong> IPM Program and <strong>for</strong> many years of<br />
commitment to helping growers in Pennsylvania<br />
and throughout the United States.<br />
Paul R. Heller (1948–2010)<br />
Professor of Entomology<br />
The Pennsylvania State University<br />
1976–2010<br />
William Merrill Jr. (1933–2003)<br />
Professor Emeritus of Plant Pathology<br />
The Pennsylvania State University<br />
1965–1999
PREFACE<br />
The Pennsylvania <strong>Integrated</strong> <strong>Pest</strong> <strong>Management</strong><br />
Program (PA IPM) is pleased to provide <strong>Integrated</strong> <strong>Pest</strong><br />
<strong>Management</strong> <strong>for</strong> <strong>Christmas</strong> <strong>Tree</strong> <strong>Production</strong>, designed<br />
to help <strong>Christmas</strong> tree growers manage pests based<br />
on sound integrated pest management principles. PA<br />
IPM is a collaboration of the Pennsylvania Department<br />
of Agriculture (PDA) and The Pennsylvania State<br />
University. This partnership has resulted in excellent<br />
service to Pennsylvania growers as well as to growers<br />
in surrounding states. The institutional collaboration<br />
embodied by PA IPM allows us to draw on Department<br />
of Agriculture technical experts and Penn State faculty<br />
and extension educators.<br />
This manual is part of an educational program that<br />
was initiated in 1985 by Dr. William Merrill Jr., professor<br />
of plant pathology, <strong>for</strong> the thriving Pennsylvania <strong>Christmas</strong><br />
tree industry. Dr. William Merrill and Ms. Nancy<br />
Wenner, along with other personnel from the Departments<br />
of Plant Pathology, Horticulture, and Entomology,<br />
organized the fi rst <strong>Christmas</strong> <strong>Tree</strong> <strong>Pest</strong> <strong>Management</strong><br />
Short Course held at Penn State University Park. Over<br />
the years, the course has evolved but is still held each<br />
winter in State College to provide growers with handson<br />
experiences in diagnosing the common insect and<br />
disease problems on <strong>Christmas</strong> trees.<br />
In 1991, Penn State entomologist Dr. Paul Heller<br />
initiated the <strong>Christmas</strong> <strong>Tree</strong> Landscape <strong>Pest</strong> <strong>Management</strong><br />
Newsletter. The newsletter was mailed to county<br />
extension offi ces and interested growers on a regular<br />
basis. PDA entomologists Rayanne Lehman and Dr. Karl<br />
Valley began contributing scouting in<strong>for</strong>mation to the<br />
newsletter in 1992. PDA staff continued the scouting<br />
program with support from PDA by providing weekly<br />
scouting reports <strong>for</strong> growers. The scouting report is<br />
still produced weekly during the growing season with<br />
support from PDA and growers.<br />
To enhance this educational outreach, and in<br />
response to the needs of <strong>Christmas</strong> tree growers <strong>for</strong><br />
pest control in<strong>for</strong>mation pertinent to Pennsylvania, a<br />
committee comprising scientists, regulators, extension<br />
educators, and growers was assembled to discuss<br />
their needs. One need was a resource customized <strong>for</strong><br />
Pennsylvania conditions that describes pests and their<br />
management. An advisory body was assembled to help<br />
design and review the manual, and several experts<br />
contributed text and photos.<br />
We hope this manual will encourage growers<br />
to develop their own IPM programs and train their<br />
employees to become familiar with IPM practices.<br />
Funding and support to write and review this<br />
manual were provided by the Pennsylvania Department<br />
of Agriculture, The Pennsylvania State University,<br />
and through grant support from the Environmental<br />
Protection Agency Region 3.<br />
—Cathy Thomas, IPM Coordinator,<br />
Pennsylvania Department of Agriculture<br />
—Ed Rajotte, Professor of Entomology and<br />
Penn State IPM Coordinator<br />
HOW TO USE THIS MANUAL<br />
The purpose of this manual is to help growers identify,<br />
monitor, and control disease and insect pests affecting<br />
<strong>Christmas</strong> trees. The pests included are common on<br />
<strong>Christmas</strong> trees in the Mid-Atlantic and Northeast<br />
regions of the United States; however, many of these<br />
pests can be found in other regions of the United<br />
States. This manual is intended <strong>for</strong> fi eld use by any level<br />
of grower, whether professional or hobbyist. Unfamiliar<br />
terms are defi ned in the glossary at the back of the<br />
manual.<br />
Steps <strong>for</strong> using this manual to identify a pest<br />
problem:<br />
1. Identify the tree species.<br />
2. Refer to Appendix A: <strong>Pest</strong> and Disease Photo<br />
Chart. This chart has a series of thumbnail photos<br />
corresponding to pests or abiotic problems. Select<br />
the photo(s) that best matches symptomatic plants<br />
and refer to the appropriate “fact sheet(s)” <strong>for</strong> additional<br />
in<strong>for</strong>mation.<br />
Each pest fact sheet provides useful in<strong>for</strong>mation,<br />
including hosts affected, damage potential,<br />
symptoms and signs, similar symptoms, calendar<br />
of activities, identifi cation tips, biology and life<br />
cycle, monitoring and management strategies,<br />
control options, and next crop/prevention strategies.<br />
Photographs contained in the fact sheets show<br />
the symptoms and life stages of the pests as seen<br />
throughout the growing season.<br />
Seasonal calendars have been included to serve<br />
as guides <strong>for</strong> scouting and controlling pests; growing<br />
degree days have been included when available.<br />
However, the best method <strong>for</strong> detection and control is<br />
still personal observation. Guidelines <strong>for</strong> scouting can<br />
be found on page 9 of this manual. Additionally, several<br />
templates (Growing Degree Day Record, Scouting Record,<br />
and <strong>Pest</strong>icide Record) are located in the appendix.<br />
These templates can be copied to help with yearly<br />
record keeping.<br />
<strong>Christmas</strong> tree scouting updates can be found at<br />
ento.psu.edu/extension/christmas-trees/scoutingreports<br />
starting in March and continuing weekly<br />
through June. Additional reports are included during<br />
the month of August. Past reports starting in 2000 are<br />
available <strong>for</strong> historical perspective.<br />
INTEGRATED<br />
PEST<br />
MANAGEMENT<br />
<strong>for</strong><br />
CHRISTMAS<br />
TREE<br />
PRODUCTION<br />
A GUIDE FOR<br />
PENNSYLVANIA<br />
GROWERS
ACKNOWLEDGMENTS<br />
Project Coordinators<br />
Cathy E. Thomas, PA IPM Coordinator<br />
Pennsylvania Department of Agriculture<br />
Sarah Pickel, Education Specialist<br />
Pennsylvania Department of Agriculture<br />
Contributors<br />
Amber Brunskill, IPM Curriculum Development Assistant<br />
Penn State Department of Entomology<br />
Rayanne D. Lehman, Entomologist (Retired)<br />
Pennsylvania Department of Agriculture<br />
Karen Najda, Plant Inspector, Region VII<br />
Pennsylvania Department of Agriculture<br />
Tracey N. Olson, Plant Pathologist<br />
Pennsylvania Department of Agriculture<br />
Sarah Pickel, Education Specialist<br />
Pennsylvania Department of Agriculture<br />
Brian Schildt, IPM Scouting Consultant<br />
Pennsylvania Department of Agriculture<br />
Cathy E. Thomas, PA IPM Coordinator<br />
Pennsylvania Department of Agriculture<br />
Reviewers<br />
Steve Derstine, Farm Manager<br />
J. C. Hill <strong>Tree</strong> Farms, Inc.<br />
Jim Fogarty<br />
Halabura <strong>Tree</strong> Farm<br />
Rayanne D. Lehman, Entomologist (Retired)<br />
Pennsylvania Department of Agriculture<br />
Kimberly J. Miller, Plant Inspector, Region III<br />
Pennsylvania Department of Agriculture<br />
Karen Najda, Plant Inspector, Region VII<br />
Pennsylvania Department of Agriculture<br />
Tracey N. Olson, Plant Pathologist<br />
Pennsylvania Department of Agriculture<br />
Ed Rajotte, Professor of Entomology and<br />
PA IPM Coordinator<br />
Penn State Department of Entomology<br />
Linda L. Signarovitz, Plant Inspector (Retired)<br />
Pennsylvania Department of Agriculture<br />
Advisers<br />
Timothy M. Abbey, Extension Educator<br />
Penn State Cooperative Extension, Capital Area<br />
Ricky Bates, Professor of Horticulture<br />
Penn State Department of Horticulture<br />
Sandy Gardosik, Entomologist<br />
Pennsylvania Department of Agriculture<br />
Ed Rajotte, Professor of Entomology and PA IPM<br />
Coordinator<br />
Penn State Department of Entomology<br />
Linda L. Signarovitz, Plant Inspector (Retired)<br />
Pennsylvania Department of Agriculture<br />
A special thanks to:<br />
George Perry, Extension Educator (Retired), Penn State<br />
Cooperative Extension, Schuylkill and Berks Counties,<br />
<strong>for</strong> his many years of dedicated service to Pennsylvania<br />
<strong>Christmas</strong> tree growers<br />
PA IPM Program Staff<br />
Ed Rajotte, Professor of Entomology and PA IPM<br />
Coordinator<br />
Penn State Department of Entomology<br />
Cathy E. Thomas, PA IPM Coordinator<br />
Pennsylvania Department of Agriculture<br />
Kristie Auman-Bauer, PA IPM Public Relations and<br />
Outreach Coordinator<br />
Penn State Department of Entomology<br />
David Biddinger, <strong>Tree</strong> Fruit Entomologist and<br />
Biocontrol Specialist<br />
Penn State Department of Entomology<br />
Amber Brunskill, Curriculum Development Assistant<br />
Penn State Department of Entomology<br />
Wade Esbenshade, IPM and Biocontrol Specialist<br />
Pennsylvania Department of Agriculture<br />
Lyn Garling, PA IPM Manager of Programs<br />
Penn State Department of Entomology<br />
Rhonda Griffi n, IPM Home Healthcare Educator<br />
Penn State Cooperative Extension in Philadelphia<br />
County<br />
Dion Lerman, Environmental Health Programs Specialist<br />
Penn State Cooperative Extension in Philadelphia<br />
County<br />
Cathy Nardozzo, PA IPM Webmaster<br />
Penn State Department of Entomology<br />
Michelle Niedermeier, Community IPM and<br />
Environmental Health Program Coordinator<br />
Penn State Cooperative Extension in Philadelphia<br />
County<br />
Sarah Pickel, Education Specialist<br />
Pennsylvania Department of Agriculture<br />
Brian Schildt, IPM Scouting Consultant<br />
Pennsylvania Department of Agriculture
CONTENTS<br />
<strong>Integrated</strong> <strong>Pest</strong> <strong>Management</strong> Basics ................ 5<br />
Step 1. Preparing <strong>for</strong> IPM: Planning and<br />
Prevention ....................................................................6<br />
Step 2. Identifi cation and Understanding of<br />
<strong>Pest</strong>s and Problems ......................................................7<br />
Insects and Mites ................................................7<br />
Diseases (Fungi and Nematodes) ........................8<br />
Vertebrates (Mammals and Birds) .......................8<br />
Weeds .................................................................8<br />
Environmental Factors .........................................8<br />
Step 3. Monitoring <strong>Tree</strong>s <strong>for</strong> <strong>Pest</strong> Populations ..............9<br />
Growing Degree Days/Temperature .....................9<br />
Soil Temperature................................................10<br />
Personal Monitoring Kit ....................................10<br />
Monitoring Tips .................................................11<br />
Trapping ...........................................................11<br />
Diagnosis ..........................................................11<br />
Step 4. Setting an Action Threshold ............................12<br />
Step 5. Selecting Control Options ...............................12<br />
Cultural and Mechanical Control .......................12<br />
Biological Control ..............................................13<br />
Chemical Control ...............................................13<br />
Step 6. Evaluating Results of Control Actions .............16<br />
Pennsylvania Conifer Certifi cation and<br />
Quarantine Guidelines ...............................................16<br />
Growing Degree Day Ranges <strong>for</strong><br />
<strong>Christmas</strong> <strong>Tree</strong> <strong>Pest</strong>s ...................................................18<br />
Insect and Mite Calendar ..........................................19<br />
<strong>Pest</strong> Fact Sheets ................................................ 21<br />
Needle Discoloration and Injury .................................22<br />
Bagworm...........................................................23<br />
Balsam Twig Aphid ............................................26<br />
Cooley Spruce Gall Adelgid on Douglas-Fir ......29<br />
Cryptomeria Scale .............................................31<br />
Cyclaneusma Needle Cast .................................34<br />
Douglas-Fir Needle Midge .................................37<br />
Elongate Hemlock Scale ....................................39<br />
Eriophyid Rust and Sheath Mites ......................42<br />
Gypsy Moth .......................................................45<br />
Lophodermium Needle Cast ..............................48<br />
Pine Needle Scale ..............................................51<br />
Pine Sawfl ies .....................................................53<br />
Ploioderma Needle Cast ....................................56<br />
Red-band (Dothistroma) Needle Blight .............58<br />
Rhabdocline Needle Cast ..................................60<br />
Rhizosphaera Needle Cast.................................63<br />
Spruce Needle Rust ...........................................65<br />
Spruce Spider Mite ............................................67<br />
Swiss Needle Cast .............................................70<br />
Shoot and Branch Injury .............................................72<br />
Atropellis Canker ...............................................73<br />
Balsam Woolly Adelgid ......................................75<br />
Botrytis Blight ...................................................78<br />
Cooley Spruce Gall Adelgid on Spruce ...............80<br />
Diplodia (Sphaeropsis) Tip Blight of Pines and<br />
Other Conifers ...................................................82<br />
Eastern Pine Weevil ...........................................84<br />
Eastern Spruce Gall Adelgid ..............................86<br />
Gall Rusts ..........................................................88<br />
Pales Weevil ......................................................90<br />
Pine Bark Adelgid ..............................................92<br />
Pine Shoot Beetle ..............................................94<br />
Striped Pine Scale ..............................................97<br />
White Pine Blister Rust ......................................99<br />
White Pine Weevil ...........................................101<br />
Stem and Root Injury/<strong>Tree</strong> Mortality ........................106<br />
Armillaria Root Rot .........................................107<br />
Bark and Engraver Beetles ..............................109<br />
Phytophthora Root Rot....................................111<br />
Pine Root Collar Weevil ...................................113<br />
Pine Wilt Disease .............................................116<br />
White Grubs ....................................................118<br />
Zimmerman Pine Moth....................................120<br />
Other Types of Damage to Conifers ..........................122<br />
Chemical Damage ...........................................123<br />
Environmental Damage ...................................124<br />
Mechanical Damage ........................................126<br />
Vertebrate <strong>Pest</strong>s ..............................................128<br />
<strong>Pest</strong>icide In<strong>for</strong>mation ..................................... 130<br />
Fungicides, Herbicides, Insecticides, and Miticides ...131<br />
<strong>Pest</strong>icide Resistance Issues .......................................131<br />
<strong>Pest</strong>icide Safety ........................................................131<br />
Appendixes ..................................................... 133<br />
A. <strong>Pest</strong> and Disease Photo Chart ............................134<br />
B. Biological Controls Photo Chart .........................144<br />
C. Conifer Species ...................................................148<br />
D. Seasonal Monitoring Guide ................................150<br />
E. Insect Trap Use and Construction .......................154<br />
F. Scouting Record Template <strong>for</strong><br />
Common Pine <strong>Pest</strong>s ............................................160<br />
G. Scouting Record Template <strong>for</strong><br />
Common Spruce <strong>Pest</strong>s ........................................164<br />
H. Scouting Record Template <strong>for</strong><br />
Common True Fir and Douglas-Fir <strong>Pest</strong>s .............168<br />
I. Growing Degree Day and Temperature<br />
Record Template .................................................172<br />
J. <strong>Pest</strong>icide Application Record Template ...............174<br />
K. Contact In<strong>for</strong>mation ...........................................176<br />
L. Lists of <strong>Pest</strong>s.......................................................182<br />
—<strong>Pest</strong>s in Alphabetical Order ............................183<br />
—<strong>Pest</strong>s by Host Genus .......................................184<br />
Bibliography .................................................... 185<br />
Glossary ........................................................... 199
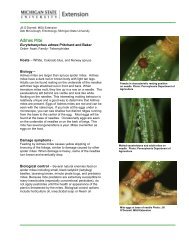
INTEGRATED<br />
PEST MANAGEMENT BASICS<br />
<strong>Christmas</strong> tree growers everywhere face obstacles<br />
to growing healthy trees. Perhaps the largest<br />
challenge is controlling the pests that feed on<br />
and live off of conifers. The aim of this manual<br />
is to help growers combat their pest problems<br />
by using integrated pest management (IPM)<br />
methods. IPM is a multilayered approach to<br />
keeping pests and pest damage to a minimal level<br />
with the smallest cost to health, environment,<br />
and budget.<br />
IPM attempts to improve on some of the<br />
common problems with traditional <strong>Christmas</strong><br />
tree pest control. One problem is improper timing<br />
of pesticides. Traditionally, growers may use<br />
two unsuccessful timing methods: calendar spraying<br />
and reaction spraying. Calendar spraying is<br />
making pesticide applications at the same time<br />
every year regardless of the actual pest situation.<br />
Reaction spraying is making a spray immediately<br />
upon discovery of a pest problem. Both of these<br />
methods are generally incorrect because they<br />
may not coincide with the target pest’s vulnerable<br />
life stage.<br />
Another problem IPM attempts to correct is<br />
the overuse of broad-spectrum, or nonspecifi c,<br />
pesticides. Broad-spectrum pesticides kill a wide<br />
range of insects, not just the target pests. Benefi<br />
cial insects that help keep pest populations in<br />
check may be eliminated by these harsh pesticides.<br />
If spray timing is not correct, growers may<br />
make multiple broad-spectrum applications and<br />
possibly still not get control. Using the steps of<br />
IPM addressed on the following pages, growers<br />
should be able to implement safer, more effective<br />
pest control.
STEP 1. Preparing <strong>for</strong> IPM:<br />
Planning and Prevention<br />
IPM practices are generally aimed at an<br />
established crop, but some pest prevention<br />
can occur be<strong>for</strong>e the crop is even planted.<br />
Most growers know what species they<br />
intend to grow at their location. However,<br />
since a tree grown in a suitable location is<br />
a good defense against pests, consider the<br />
following points be<strong>for</strong>e planting:<br />
• Property assessment. Identifying the<br />
challenges to growing trees on a property<br />
will help growers determine the<br />
best locations <strong>for</strong> tree blocks. Assessing<br />
the property may include collecting and<br />
testing soil samples, surveying property<br />
slope and topography, and determining<br />
water drainage rates and sunlight levels.<br />
Historical meteorological in<strong>for</strong>mation<br />
and knowledge of past crops can be useful.<br />
Talking to area growers about possible pest<br />
problems (insect, mite, disease, mammal,<br />
etc.) to expect would also be benefi cial.<br />
• <strong>Tree</strong> species. Selecting tree species best<br />
suited to the property is the next step.<br />
This will involve research and may mean<br />
that the site is not appropriate <strong>for</strong> the<br />
intended species. By using printed publications,<br />
Web resources, and in<strong>for</strong>mation<br />
from other growers and seedling nurseries,<br />
growers can predict the success of the<br />
intended crop. For instance, knowing that<br />
Colorado blue spruce may grow best in a<br />
fl at, sometimes wet fi eld can help a grower<br />
decide not to plant Fraser fi r, which could<br />
be susceptible to root rot issues in this<br />
same location.<br />
• Seed source. In addition to choosing<br />
tree species, growers may have options<br />
<strong>for</strong> the seedlings based on seed source.<br />
Seed sources generally refer to different<br />
geographical regions where the trees grow<br />
naturally. The trees resulting from these<br />
seeds may be adapted to certain growing<br />
conditions or may show some pest resistance<br />
in addition to foliage and growth<br />
attributes. Growers selecting seedlings<br />
from a particular seed source <strong>for</strong> its pest<br />
resistance need to understand that resistance<br />
characteristics may change when<br />
the tree is grown outside its natural range.<br />
• Planting. The success of a tree block has a<br />
lot to do with proper planting of seedlings<br />
and transplants. Consider the following<br />
factors when planting:<br />
— Spacing. Adequate spacing between<br />
the trees will provide good air circulation,<br />
which can prevent disease<br />
development and allow <strong>for</strong> good spray<br />
coverage during pesticide applications.<br />
Other factors to consider when spacing<br />
trees are the minimum requirements<br />
<strong>for</strong> mowing, harvesting, and shearing<br />
equipment. A spacing of 5½ feet by<br />
5½ feet or 6 feet by 6 feet will allow <strong>for</strong><br />
fewer trees than a spacing of 4 feet by<br />
4 feet, but the trees with wider spacing<br />
will be healthier through harvest time.<br />
Spacing needs will vary depending on<br />
the fi nal desired age of the trees in a<br />
block.<br />
— Depth. The depth at which seedlings<br />
are planted will greatly affect tree<br />
health. Planting trees so that the root<br />
collar is at or just slightly below the soil<br />
line and not planted too deep or too<br />
shallow will be a factor in preventing<br />
pest attack.<br />
— Method. Some planting methods<br />
are more prone to future problems.<br />
Mechanical planters may encourage<br />
J-rooting. In this situation, roots do<br />
not spread out but actually grow in a<br />
“J” shape (Fig. 1). As a result, trees are<br />
unable to establish a good root system,<br />
which after several years will lead to<br />
poor tree health. J-rooted trees are also<br />
more susceptible to insect and disease<br />
problems.<br />
Figure 1. J-rooted tree resulting from improper<br />
planting. Courtesy of Tracey Olson, PDA<br />
IPM FOR CHRISTMAS TREE PRODUCTION ............................................................................................................................................................................................................................................. 6
STEP 2. Identifi cation and<br />
Understanding of <strong>Pest</strong>s and<br />
Problems<br />
After the <strong>Christmas</strong> tree block is planted,<br />
the focus of an IPM approach will be on<br />
preventing losses due to pest damage. The<br />
term “pest” may include any organism—<br />
diseases, insects, mites, nematodes, mammals,<br />
birds, or weeds—that is detrimental<br />
to the health of the tree. Environmental<br />
factors such as air pollution may also cause<br />
damage. Knowing which pest or what factor<br />
is causing damage to a tree is essential to<br />
fi nding a means of stopping or controlling<br />
that problem.<br />
Insects and Mites<br />
The largest groups of <strong>Christmas</strong> tree pests<br />
are two classes of arthropod pests, or organisms<br />
with jointed body parts and an exoskeleton.<br />
These two classes are the insects<br />
and the arachnids. Arachnids include mites,<br />
spiders, and ticks, but only the mites can<br />
be detrimental to tree health. Experienced<br />
growers are familiar with the insect and<br />
mite pests that may affect their trees. They<br />
have, or know where to fi nd, in<strong>for</strong>mation<br />
about the life cycle and habits of these pests<br />
in order to determine the best method and<br />
timing <strong>for</strong> both detection and control, if<br />
necessary (Fig. 2).<br />
For example, spider mites (Fig. 3) and<br />
(eriophyid) rust mites feed openly on tree<br />
foliage without any extra protection. It is<br />
easier to control these pests than one that<br />
spends most of its life covered by a protective<br />
coating, such as pine needle scale. In<br />
addition to the mites, pests that feed openly<br />
include aphids, gypsy moths, and sawfl ies.<br />
The larger group of insect and mite pests<br />
includes those that are only exposed <strong>for</strong> a<br />
short time during their life cycle. Timing<br />
sprays to target susceptible life stages is critical<br />
<strong>for</strong> effective control. <strong>Pest</strong>s that fall into<br />
this category include all the scale insects,<br />
most adelgids, bagworm (Fig. 4), weevils,<br />
bark beetles, and midges.<br />
Knowing these and other insects is essential<br />
<strong>for</strong> growers to protect their trees from<br />
pest infestation. In addition, recognizing<br />
which insects and mites are benefi cial and<br />
taking steps to avoid killing these organisms<br />
can limit or even eliminate the need <strong>for</strong><br />
chemical control measures.<br />
Stinging insects, such as wasps and bees,<br />
are also a major concern on <strong>Christmas</strong> tree<br />
farms, especially during summer mowing<br />
and fi eld maintenance. These insects are<br />
frequently attracted to trees infested with<br />
aphids and scale insects. Recognizing both<br />
the pest and the stinging insects and understanding<br />
their biology are important when<br />
considering employee safety.<br />
Figure 2. Balsam twig aphid life cycle. Courtesy of Sarah Pickel, PDA (photos courtesy of PDA)<br />
Figure 3. Spruce spider mite<br />
feeding openly on tree foliage.<br />
Courtesy of Rayanne D. Lehman,<br />
PDA<br />
Figure 4. Protective casing<br />
containing bagworms. Courtesy<br />
of Rayanne D. Lehman, PDA<br />
IPM BASICS .............................................................................................................................................................................................................................................................................................. 7
Figure 5. Rhabdocline needle<br />
cast symptoms on Douglas-fi r.<br />
Courtesy of Rayanne D. Lehman,<br />
PDA<br />
Figure 6. Reddish-brown<br />
splotches, symptoms of Rhabdocline,<br />
found in late winter.<br />
Courtesy of Tracey Olson, PDA<br />
Figure 7. Buck rub on a young<br />
fi r. Courtesy of Rayanne D.<br />
Lehman, PDA<br />
Diseases (Fungi and Nematodes)<br />
Numerous diseases of <strong>Christmas</strong> trees are<br />
caused by fungi and nematodes, with the<br />
majority being caused by fungi. Fungi are<br />
simple organisms that survive in soil, water,<br />
and dead or living organic material. Cool<br />
temperatures, high moisture, and low air<br />
circulation will encourage fungal development.<br />
In <strong>Christmas</strong> tree plantations, these<br />
factors often occur together, making disease<br />
development common. Symptoms include<br />
swelling or shrinking of the wood tissue,<br />
discolored or de<strong>for</strong>med needles, early needle<br />
drop, and wilted foliage. The disease types<br />
are referred to as cankers, galls, needle casts,<br />
rusts, rots, and blights.<br />
Fungal diseases spread from tree to tree<br />
by microscopic spores. At a specifi c stage in<br />
disease development, mature fruiting bodies<br />
on the diseased host release the spores.<br />
Wind, rain, or other physical contact will<br />
spread these spores to other trees, initiating<br />
a new disease cycle. Growers should be<br />
aware of the diseases that may affect their<br />
trees and have an understanding of the<br />
symptoms (Figs. 5 and 6).<br />
Unlike the insect and mite pests,<br />
diseases are not usually controlled—they<br />
are prevented. Fungicides will not kill the<br />
fungus but only protect the plant tissue from<br />
infection. There<strong>for</strong>e, most fungicides must<br />
be applied be<strong>for</strong>e spores would contact the<br />
healthy tissue.<br />
Less common are the diseases caused by<br />
nematodes. Nematodes are microscopic,<br />
simple worms. They damage plants by<br />
sucking out the contents of plant cells. In<br />
the case of pine wilt disease, a wood-boring<br />
beetle transfers nematodes to the tree.<br />
Vertebrates (Mammals and Birds)<br />
Vertebrates are animals with backbones.<br />
The vertebrates of concern <strong>for</strong> <strong>Christmas</strong><br />
trees are birds and mammals, especially deer<br />
and rodents. Birds may damage tree leaders<br />
by perching on the tender growth, resulting<br />
in broken or bent leaders. Deer may severely<br />
damage shoot tips by feeding on them.<br />
Bucks can also cause injury by rubbing their<br />
antlers on the main trunk (Fig. 7). Rodents<br />
may kill trees by girdling the lower trunk as<br />
they feed on the bark.<br />
Weeds<br />
Weeds are undesirable plants that interfere<br />
with the growth of a crop such as <strong>Christmas</strong><br />
trees. Generally herbaceous, they reproduce<br />
and spread through seeds and rhizomes<br />
(underground stems). Weeds compete with<br />
trees <strong>for</strong> soil nutrients, water, and sunlight<br />
(Fig. 8). They inhibit good air circulation<br />
between trees—a contributing factor <strong>for</strong><br />
disease development. Weeds also hinder<br />
the movement of people and equipment<br />
through and between rows, making good<br />
pesticide coverage diffi cult. Through good<br />
mowing practices and the assistance of<br />
herbicides, growers can control weeds to a<br />
manageable level.<br />
Weed control will not be covered in this<br />
manual. In<strong>for</strong>mation on weeds can be found<br />
in the Penn State publication Controlling<br />
Weeds in Nursery and Landscape Plantings<br />
by Dr. Larry Kuhns. Growers can also look<br />
to their state land-grant universities <strong>for</strong> additional<br />
weed resources.<br />
Environmental Factors<br />
Injury to <strong>Christmas</strong> trees is not solely a result<br />
of living organisms. Environmental factors<br />
can also have a negative effect. Damage<br />
from severe weather can be equally as bad<br />
or worse than a disease or insect problem.<br />
Extreme cold, wind, or hail (Fig. 9) can<br />
cause needle discoloration, shoot dieback, or<br />
lesions on stems and twigs. Some unnatural<br />
environmental factors also exist. <strong>Pest</strong>icides<br />
and fertilizers applied at the wrong time of<br />
year or in the wrong amounts will also damage<br />
the trees. Air pollution, although hard to<br />
identify, may also harm trees.<br />
Figure 8. Common milkweed, a familiar nursery<br />
weed in the Mid-Atlantic and Northeast United<br />
States. Courtesy of Theodore Webster, USDA<br />
Agricultural Research Service, Bugwood.org<br />
(#1552251)<br />
Figure 9. Hail damage<br />
on eastern white<br />
pine. Courtesy of<br />
Rayanne D. Lehman,<br />
PDA<br />
IPM FOR CHRISTMAS TREE PRODUCTION ............................................................................................................................................................................................................................................. 8
STEP 3. Monitoring <strong>Tree</strong>s <strong>for</strong> <strong>Pest</strong><br />
Populations<br />
Monitoring is the key to any successful IPM<br />
plan. On farms not using IPM, the process<br />
of pest control usually involves pesticide<br />
applications based on a calendar date. These<br />
types of applications are made regardless of<br />
the verifi ed presence of the target pest. Another<br />
type of pesticide application on farms<br />
not using IPM is the “see and spray” method:<br />
spray only when the pest or damage is seen.<br />
At times, applications are made based only<br />
on damage, not on actual pest presence. IPM<br />
looks at pest management differently. Monitoring<br />
to determine whether there is a need<br />
to take action is more effective than either<br />
of the methods above.<br />
Monitoring provides growers with an<br />
accurate picture of pest situations on their<br />
<strong>Christmas</strong> tree farms. Monitoring includes<br />
the use of traps that catch pests to indicate<br />
their presence as well as direct observation<br />
by a scout. The process involves regular,<br />
close inspection of the trees on the farm <strong>for</strong><br />
symptoms or signs of pest activity (Fig. 10).<br />
“Symptoms” refer to the damage or evidence<br />
of activity, such as yellowed needles<br />
or wilted shoots; “signs” refer to the actual<br />
organism causing the damage, such as black<br />
fruiting bodies on the underside of a needle<br />
or a bagworm case hanging from a branch.<br />
By inspecting <strong>for</strong> these symptoms and signs,<br />
noting the stage of development, and<br />
evaluating the level of infestation or infection,<br />
growers can create an individualized,<br />
accurate, and timely plan of action <strong>for</strong> each<br />
fi eld. Monitoring can be done by a farm employee<br />
whose time is committed to scouting<br />
activity or by a professional scout hired as a<br />
consultant. The key is to scout regularly, not<br />
just once or twice a season.<br />
Figure 10. Scout conducting his<br />
weekly activities. Courtesy of<br />
Cathy Thomas, PDA<br />
Growing Degree Days/Temperature<br />
One method of monitoring specifi c pest populations<br />
is growing degree day (GDD) tracking.<br />
For insects, mites, and plants, development is<br />
infl uenced by daily heat accumulations from<br />
ambient air. (Disease progression is based<br />
on other factors such as host plant development,<br />
humidity, leaf wetting, etc.; there<strong>for</strong>e,<br />
the GDD method does not apply.) Tracking<br />
heat accumulation by monitoring daily air<br />
temperatures has proved useful in predicting<br />
the appearance of certain pest life stages.<br />
The growing degree day method uses the<br />
daily average temperature (using low and<br />
high temperatures <strong>for</strong> 24 hours) to determine<br />
the heat unit exposure of the pest or<br />
plant. The GDDs accumulate daily until the<br />
required amount of heat <strong>for</strong> an event (hatch,<br />
emergence, pupation, etc.) is reached. This<br />
is not a specifi c number, but a range. For<br />
instance, spruce spider mite overwintering<br />
eggs begin to hatch around 50–121 GDDs.<br />
A scout can effectively monitor <strong>for</strong> a pest by<br />
knowing the range of GDDs <strong>for</strong> the target<br />
stage of each pest. Known GDD ranges <strong>for</strong><br />
many <strong>Christmas</strong> tree pests can be found in<br />
the Growing Degree Days Chart on page 18<br />
and on each individual pest fact sheet.<br />
As a general rule, GDD recording should<br />
begin on March 1. Although each organism<br />
has a unique base temperature, most GDD<br />
calculations use a base temperature of 50°F.<br />
The base temperature is the minimum temperature<br />
required by an organism <strong>for</strong> development<br />
to proceed. Temperature should be<br />
tracked using a minimum (low)/maximum<br />
(high) thermometer capable of storing readings<br />
<strong>for</strong> several days. After calculating each<br />
day’s GDD value, add it to the accumulated<br />
total. Never include negative numbers.<br />
GDD FORMULA<br />
Low Temperature + High Temperature – 50°F = Daily GDD<br />
2<br />
(Average temperature (base<br />
<strong>for</strong> a 24-hour period) temp.)<br />
IPM BASICS .............................................................................................................................................................................................................................................................................................. 9
Figure 11. A minimum/maximum<br />
thermometer collecting<br />
temperature data in a fi eld.<br />
Courtesy of Cathy Thomas, PDA<br />
Figure 12. A probe thermometer<br />
measuring soil temperature<br />
at a depth of approximately<br />
2 inches. Courtesy of Cathy<br />
Thomas, PDA<br />
DAILY WEATHER LOG<br />
Air Temperature Daily<br />
GDD<br />
Running<br />
Date Time Low High Ave. GDD Total<br />
3/24 5:30 pm 45°F 73°F 59°F 9 9<br />
3/25 5:30 pm 35°F 55°F 45°F 0 9<br />
3/26 5:30 pm 46°F 60°F 53°F 3 12<br />
March 24<br />
45 + 73 = 118 = 59 – 50 = 9 GDD Count positive number.<br />
2 2<br />
March 25<br />
35 + 55 = 90 = 45 – 50 = -5 GDD Do not add negative<br />
2 2 numbers.<br />
March 26<br />
46 + 60 = 106 = 53 – 50 = 3 GDD Add positive number<br />
2 2 to total.<br />
Remember that temperatures may differ<br />
in different blocks. A tree block on a southfacing<br />
slope will experience higher temperatures<br />
than a block on a north-facing slope,<br />
which can result in several day differences<br />
in pest development.<br />
Using minimum/maximum thermometers<br />
(Fig. 11) is the least expensive method of<br />
gathering data to calculate GDDs. Several<br />
other types of equipment will gather GDD in<br />
the fi eld. These are commonly called biophenometers,<br />
data loggers, or weather monitors.<br />
The prices vary, but they are generally more<br />
expensive than a simple minimum/maximum<br />
thermometer. Weather services, both paid<br />
and free, can also provide required data.<br />
Skybit is a subscriber-based service located<br />
in Pennsylvania (see www.skybit.com). The<br />
National Weather Service (NOAA) provides<br />
daily low and high temperatures from<br />
weather stations. NOAA data will not be<br />
specifi c to your area and should not replace<br />
on-farm temperature collection.<br />
Soil Temperature<br />
Research conducted in Pennsylvania on<br />
white pine weevil (Pissodes strobi) has<br />
shown that collection of soil temperatures<br />
is equally or more effective than GDD in<br />
determining the weevil’s spring emergence<br />
time. Temperatures were taken with a probe<br />
thermometer from soil beneath a tree in<br />
the target block. The probe was inserted<br />
to a depth of 2 inches on the sunny side of<br />
the tree (Fig. 12). Once the soil temperature<br />
reached 50°F, weevils could be found<br />
in traps and on tree leaders. This method<br />
may also be effective on other insects that<br />
overwinter in soil.<br />
Personal Monitoring Kit<br />
When monitoring, a scout must have a few<br />
very important tools. Keep these tools together<br />
in a backpack or bag <strong>for</strong> convenient<br />
transport to the fi eld on each visit (Fig. 13).<br />
HAND LENS<br />
This small magnifi er allows a scout to see insects,<br />
mites, and fungal fruiting bodies that<br />
would otherwise be too small to see (Fig.<br />
14). These can be purchased in magnifi cation<br />
strengths of 10X, 15X, and 20X. The<br />
15X hand lens is a good strength to use and<br />
still has a reasonably sized fi eld of view (see<br />
Appendix K <strong>for</strong> supplier in<strong>for</strong>mation).<br />
Figure 13. Scouting tools and convenient zipper<br />
pack <strong>for</strong> storage. Courtesy of Brian Schildt, PDA<br />
Figure 14. The number one<br />
tool <strong>for</strong> a scout: a hand lens.<br />
Courtesy of Tracey Olson, PDA<br />
NOTEBOOK AND PEN<br />
Use these to record symptoms, signs,<br />
population stage and level, damage severity,<br />
fi eld or weather conditions, and location.<br />
This in<strong>for</strong>mation can be a reference <strong>for</strong> the<br />
current season as well as a resource that<br />
shows pest activity from previous seasons.<br />
Suggested <strong>for</strong>ms <strong>for</strong> scouting observations<br />
and notes are included in the Appendix<br />
section of this manual.<br />
FLAGGING TAPE<br />
Use brightly colored fl agging tape to mark<br />
symptomatic trees so they can be readily<br />
located at a later date. Use a permanent<br />
marker to record specifi c in<strong>for</strong>mation on the<br />
tape. Remember to use a color other than<br />
those used <strong>for</strong> digging or cutting purposes.<br />
IPM FOR CHRISTMAS TREE PRODUCTION ........................................................................................................................................................................................................................................... 10
CLIPBOARD/FLAT SURFACE<br />
Hold a clipboard or another fl at surface<br />
under a branch to collect mites, aphids,<br />
or other insects dislodged by tapping the<br />
branch (Fig. 15). The back of a clipboard<br />
that has been painted to have one half<br />
black and one half white is very useful when<br />
beating trees to detect pests. The inside<br />
back cover of this manual is a good fl at<br />
surface to use.<br />
PRUNERS/POCKET KNIFE<br />
Growers may need to clip symptomatic<br />
twigs or branches to observe them more<br />
closely <strong>for</strong> signs of insects, mites, or disease<br />
spores. Collecting samples <strong>for</strong> identifi cation<br />
by a diagnostic expert (Fig. 16) may also be<br />
necessary.<br />
BOTTLES/PLASTIC BAGS<br />
Occasionally, growers need to collect samples<br />
to examine at a later date or send to a<br />
diagnostic laboratory. In addition to bottles<br />
and plastic bags <strong>for</strong> collecting samples,<br />
having a method of labeling the specimens<br />
(permanent marker, adhesive labels, etc.) is<br />
also helpful. Any sample collected in alcohol<br />
should be labeled with a pencil-written<br />
tag inserted into the vial.<br />
Monitoring Tips<br />
1. During the growing season, a scout<br />
should be monitoring fi elds weekly to<br />
look <strong>for</strong> evidence of pests, diseases, and<br />
other problems.<br />
2. If possible, scout on cloudy days. The<br />
muted sunlight makes it easier to observe<br />
chlorotic, or yellowed, symptoms<br />
in the fi eld. Some pests are also more<br />
visible in subdued light.<br />
3. When monitoring blocks <strong>for</strong> problems,<br />
scouts should walk through the fi eld<br />
while keeping an eye out <strong>for</strong> obvious<br />
problems. They should also select nonsymptomatic<br />
trees in a random pattern<br />
to inspect more closely. For these trees,<br />
be sure to look at the inside <strong>for</strong> discoloration.<br />
4. Prune a few twigs from the interior<br />
and lower portion of the tree <strong>for</strong> closer<br />
inspection.<br />
5. Tapping branches over a fl at surface<br />
will dislodge insects and mites, making<br />
them easier to see (use the tapping<br />
sheet at the back of this manual).<br />
Trapping<br />
Several types of insects can be monitored<br />
using traps. The traps are usually baited with<br />
pheromones (insect-produced attractants)<br />
or other chemicals (Fig. 17) and placed in<br />
the fi eld. They are designed to hold the<br />
insect until the trap can be checked by<br />
a scout. When monitored regularly, the<br />
collection data can help with the timing<br />
of control applications. Traps are used <strong>for</strong><br />
monitoring purposes, not control. For more<br />
in<strong>for</strong>mation on trapping, see Appendix E.<br />
Sticky cards are another type of monitoring<br />
tool used to trap insects. These<br />
adhesive-coated index cards, which come<br />
in various sizes and colors, may be used<br />
to monitor the emergence of small pests<br />
(Fig. 18). For example, in the case of some<br />
scale insect pests, 3-by-5-inch sticky cards<br />
are used to attract the adult male life stage.<br />
The cards are attached with clothespins<br />
to scale-infected tree limbs be<strong>for</strong>e the<br />
expected time of emergence. Cards can<br />
then be monitored regularly.<br />
Figure 18. Yellow sticky cards used to trap<br />
fl ying adult male scales. Courtesy of Cathy<br />
Thomas, PDA<br />
Diagnosis<br />
Proper identifi cation of the pests that<br />
have been found through the monitoring<br />
process is an important next step in the<br />
pest management process. With training,<br />
experience, and the use of printed and Web<br />
resources and guides, growers or scouts can<br />
usually make an accurate diagnosis. For<br />
diffi cult samples, consult an expert or a<br />
diagnostic lab. Regional inspectors <strong>for</strong> the<br />
state department of agriculture and county<br />
extension educators can be very helpful in<br />
obtaining necessary expert assistance. For<br />
contact in<strong>for</strong>mation <strong>for</strong> select agencies, see<br />
Appendix K.<br />
Figure 15. Tapping branches<br />
over a white notebook or fl at<br />
surface to dislodge and observe<br />
pests. Courtesy of Brian Schildt,<br />
PDA<br />
Figure 16. A good set of pruners.<br />
Courtesy of Joseph O’Brien,<br />
USDA Forest Service, Bugwood<br />
.org (#5044044)<br />
Figure 17. White pine weevil<br />
trap. Courtesy of Cathy Thomas,<br />
PDA<br />
IPM BASICS ............................................................................................................................................................................................................................................................................................ 11
STEP 4. Setting an Action<br />
Threshold<br />
The action or control threshold refers to the<br />
population level <strong>for</strong> a specifi c pest at which<br />
some control measure is justifi ed in order<br />
to avoid economic loss or aesthetic damage<br />
to a crop. <strong>Christmas</strong> trees can sustain some<br />
damage and still be aesthetically acceptable<br />
to consumers. However, when the damage<br />
becomes unacceptable, the crop has passed<br />
what is known as the aesthetic injury level.<br />
The action threshold will be exceeded<br />
be<strong>for</strong>e the aesthetic injury level has been<br />
reached.<br />
Because pests vary in the severity of<br />
damage they cause, action thresholds will<br />
also vary. A pest that has great potential<br />
to damage or disfi gure a tree, such as white<br />
pine weevil, will have a lower threshold<br />
than a more minor pest, such as the balsam<br />
twig aphid, <strong>for</strong> which some damage may be<br />
acceptable. Action thresholds are further<br />
discussed in the individual fact sheets found<br />
in this manual.<br />
The question of acceptable action<br />
thresholds will also vary depending on how<br />
the trees are marketed. If trees are sold as<br />
nursery stock, often referred to as balledand-burlapped<br />
(B&B) trees <strong>for</strong> the way<br />
they are dug and bound, then the issue of<br />
threshold is no longer solely determined by<br />
the grower. All nursery stock in Pennsylvania<br />
is subject to state regulation, and the<br />
level of pest tolerance <strong>for</strong> the trees is based<br />
on a regulatory threshold. These regulatory<br />
thresholds are en<strong>for</strong>ced by state or federal<br />
nursery inspectors. Some of these thresholds,<br />
such as those <strong>for</strong> pests under state or<br />
federal quarantines, are zero tolerance. Cut<br />
<strong>Christmas</strong> trees in Pennsylvania are not subject<br />
to regulation. However, if trees are to be<br />
shipped out of state, federal quarantines will<br />
be en<strong>for</strong>ced.<br />
Growers need to be aware of these<br />
regulatory issues when considering control<br />
actions on their farms. More in<strong>for</strong>mation on<br />
state regulatory requirements can be found<br />
in the Conifer Certifi cation and Quarantine<br />
Guidelines section on page 16.<br />
STEP 5. Selecting Control Options<br />
IPM does not usually rely on only one<br />
method or tactic. Often, growers employ a<br />
combination of methods to achieve a wellrounded,<br />
long-lasting management program.<br />
Cultural and Mechanical Control<br />
Best growing practices and physical methods<br />
of control are often the fi rst defense to<br />
pest issues and can be the least expensive.<br />
Examples of these controls include:<br />
• Mowing. Through consistent management<br />
of grasses and weeds by mowing,<br />
growers are able to facilitate good air<br />
circulation through tree blocks. Good air<br />
circulation helps prevent disease development<br />
and opens the lower portion of the<br />
trees to receive chemical applications, if<br />
necessary.<br />
• Pruning. In addition to shaping trees,<br />
pruning can also help eliminate some<br />
pest issues. Pruning is one way to help<br />
eliminate the galls produced by adelgids<br />
or needles infested with needle midges.<br />
Growers may eliminate some of the<br />
feeding damage produced by balsam twig<br />
aphids, bagworm, or pine sawfl ies. Also,<br />
clipping out leaders infected by white<br />
pine weevil is an effective management<br />
method. Butt-pruning, or the removal of<br />
the lowest whorl of branches, opens the<br />
bottom of the tree to provide better air<br />
circulation, weed control, and pesticide<br />
application. It also permits heating of the<br />
soil and may make the area less attractive<br />
to certain insect and rodent pests.<br />
• Culling. This is the practice of removing<br />
weakened, infected, and dying trees from<br />
the fi elds. Eliminating these reservoirs<br />
from the fi elds not only removes a source<br />
of infestation but will also allow <strong>for</strong> better<br />
air circulation among the remaining trees.<br />
It is an effective, low-cost method <strong>for</strong><br />
reducing a recently introduced pest that<br />
has the potential to cause serious damage.<br />
IPM FOR CHRISTMAS TREE PRODUCTION ........................................................................................................................................................................................................................................... 12
Biological Control<br />
Biological control, or biocontrol, is the use<br />
of living organisms (parasitoids, pathogens,<br />
predators) to control diseases, insect pests,<br />
and weeds (Fig. 19). Biocontrol plays an<br />
important role in IPM but does not defi ne<br />
it. Many IPM techniques are geared toward<br />
enhancing or preserving biocontrols. A<br />
successful IPM program combines many<br />
methods aimed at keeping pest populations<br />
below damaging levels.<br />
In <strong>Christmas</strong> trees, no biocontrol agents<br />
exist <strong>for</strong> disease, and those <strong>for</strong> weed control<br />
are poorly understood. But numerous<br />
naturally occurring parasitoids, pathogens,<br />
and predators of insects and mites are present<br />
(Fig. 20). Growers are often not aware<br />
of these natural enemies and continue to<br />
rely on insecticides to control pests. Excessive<br />
pesticide use may eliminate natural<br />
enemies, further increasing reliance on<br />
pesticides. The goal of a good IPM program<br />
is to recognize and preserve these biocontrol<br />
agents. The benefi t is often a reduction of<br />
pesticides and costs.<br />
In order <strong>for</strong> natural enemies to survive,<br />
they must not be 100 percent effi cient. A<br />
small number of pests are always needed to<br />
keep the natural enemies around. Because<br />
the pests are not eliminated, biocontrol is<br />
not as popular when dealing with a crop<br />
such as <strong>Christmas</strong> trees where aesthetics<br />
can be important. However, biocontrol<br />
agents are extremely useful if dealing with<br />
a pest that either has a high threshold of<br />
damage or can be tolerated in a low-level<br />
population <strong>for</strong> a long time.<br />
Not all pests are suitable <strong>for</strong> biocontrol.<br />
Borers, <strong>for</strong> instance, must be treated as soon<br />
as they are detected. White pine weevil<br />
has very few natural enemies, and none are<br />
known <strong>for</strong> pine root collar weevil. Some<br />
pests, such as aphids and mites, are easier to<br />
keep in check with natural enemies, but this<br />
is only applicable if regular scouting is used to<br />
monitor both the pest and benefi cial populations.<br />
By monitoring, growers can take action<br />
if the pest populations are not being kept in<br />
check by their natural enemies.<br />
Some research has been done with<br />
insect-pathogenic nematodes that grow in<br />
the bodies of their hosts (e.g., beetle larvae),<br />
eventually killing them. Neoaplectena<br />
carpocapsae (Weiser) has been extensively<br />
studied and tested, and Heterorhabditis heliothidis<br />
is receiving attention. In order <strong>for</strong> the<br />
nematode to do its job, it must occur with a<br />
bacterium since neither can survive alone.<br />
Sometimes the nematode-bacterium is not as<br />
effective as an insecticide, but that depends<br />
on the situation. The most critical factor<br />
restricting wide use of insect-pathogenic<br />
nematodes is overcoming nematode environmental<br />
sensitivity. Nematode success is<br />
greatest when moisture and temperature<br />
are at the optimum level <strong>for</strong> that location.<br />
Active research continues at universities<br />
and in private industries to increase the effectiveness<br />
of insect-pathogenic nematodes<br />
and make them a more cost-effective tool.<br />
Chemical Control<br />
When biocontrols are not suitable, not<br />
working as they should, or overwhelmed by<br />
a pest outbreak, chemical control is the next<br />
step. “Soft” or “reduced-risk” pesticides,<br />
such as insecticidal soap, ultra-fi ne horticultural<br />
oil, and neem compounds, are used<br />
<strong>for</strong> treating outbreaks that happen while<br />
managing with biocontrols. Consult with<br />
the biocontrol distributor be<strong>for</strong>e spraying to<br />
ensure compatibility.<br />
The best control plan treats the pest<br />
populations with minimal disruption to<br />
the natural enemies. This can be accomplished<br />
several ways, including alternating<br />
insecticide classes, altering spray timing, or<br />
adjusting cultural practices. Another approach<br />
is to use soft insecticides that are not<br />
harmful to benefi cial insects. Horticultural<br />
oils are effective in killing overwintering<br />
eggs and sessile insects but do little harm to<br />
natural enemies. Caterpillars, but not sawfl y<br />
larvae, can be controlled with the bacterial<br />
insecticide Bacillus thuringiensis, or Bt. Some<br />
pest-specifi c insect growth regulators (IGRs)<br />
are also effective. There are three general<br />
types of chemical control: biorational,<br />
horticultural oil, and traditional.<br />
BIORATIONALS<br />
As pesticide regulations increase and the<br />
general public gains awareness of pesticides<br />
used on <strong>Christmas</strong> tree farms, more attention<br />
is directed toward alternatives. Alternatives,<br />
otherwise known as biorational<br />
products, include insecticidal soap, pyrethrum,<br />
and rotenone (not an oil).<br />
Insecticidal Soap<br />
The insecticidal action results when fatty<br />
acids from plant oils are combined with the<br />
potassium bases to produce potassium salts<br />
of fatty acids. The inactive ingredients are<br />
water and alcohol.<br />
Figure 19. A praying mantis, a<br />
generalist predator. Courtesy of<br />
Cathy Thomas, PDA<br />
Figure 20. Multicolored Asian<br />
lady beetle larva, an aphid<br />
predator. Courtesy of Cathy<br />
Thomas, PDA<br />
IPM BASICS ............................................................................................................................................................................................................................................................................................ 13
Advantages:<br />
1. The mode of action (MOA) of insecticidal<br />
soap is physical, so pests are less<br />
likely to develop resistance. Soap acts<br />
on contact, penetrating body and cell<br />
membranes and causing quick death.<br />
2. Insecticidal soap has very few long-term<br />
negative effects on benefi cial insects.<br />
3. Insecticidal soap is relatively nontoxic<br />
to wildlife and humans.<br />
4. It can be applied using a variety of<br />
application equipment, and disposal of<br />
unused material and containers is easy.<br />
Disadvantages:<br />
1. The main drawback of using insecticidal<br />
soap is the lack of residual activity;<br />
there<strong>for</strong>e, more frequent applications<br />
are required.<br />
2. The effectiveness of insecticidal soap<br />
decreases when mixed with hard water.<br />
3. Soap will kill natural enemies on<br />
contact when plant surfaces are wet.<br />
Neem Seed Extracts<br />
Neem seed extracts are botanical or plantderived<br />
pesticides. Neem oil extracts are<br />
from seeds of the neem or margosa tree<br />
(native to Asia). Azadirachtin, one of the<br />
active ingredients in neem oil, has growthregulating<br />
properties. Neem oil has more<br />
than 12 other active ingredients that act<br />
as egg-laying deterrents, feeding inhibitors,<br />
growth regulators, repellents, sterilizing<br />
agents, or toxins.<br />
Advantage:<br />
1. Various neem products affect more than<br />
170 species of insects, mites, and nematodes<br />
that infest households, humans,<br />
livestock, plants, and stored products.<br />
Disadvantages:<br />
1. The raw neem oil has an unpleasant<br />
garlic-sulfur smell, which is often<br />
eliminated in commercial production.<br />
2. Natural enemies of plant pests are<br />
slightly affected by these products.<br />
3. Effectiveness may be questionable.<br />
Bacillus thuringiensis (Bt)<br />
Bt is a bacterial or microbial insecticide<br />
that attacks the gut of Lepidoptera larvae.<br />
The caterpillars ingest the Bt spores, which,<br />
in the alkaline conditions of the insect’s<br />
gut, release toxins. This causes the insect’s<br />
own digestive juices to attack the gut wall,<br />
creating holes that allow the contents to<br />
poison the body. Death may take a few days,<br />
but the insect stops feeding soon after the<br />
Bt is ingested. It is best applied when the<br />
caterpillars are young. Other <strong>for</strong>mulations<br />
of Bt exist that target other insect groups,<br />
but none have been successfully used in<br />
<strong>Christmas</strong> tree production. Bt is used to<br />
control caterpillars and has no effect on<br />
sawfl y larvae.<br />
Bacillus popilliae<br />
Better known as milky spore disease, Bacillus<br />
popilliae was the fi rst commercially introduced<br />
microbial insecticide. It has been<br />
used <strong>for</strong> many years to control Japanese<br />
beetle larvae in soil. The spores will remain<br />
dormant in the soil until future generations<br />
of Japanese beetle larvae are present. It is<br />
not effective against other white grubs.<br />
HORTICULTURAL OILS<br />
Horticultural oils work in two ways. The<br />
fi rst MOA is suffocation. When the oil coats<br />
the insect’s body, it prevents the exchange<br />
of gases in the pest. Eggs are also affected in<br />
this manner. The second MOA is penetration<br />
of the insect’s external membranes and<br />
subsequent entrance into the individual<br />
cells, where cell functions are disrupted.<br />
Both dormant oil and the highly refi ned<br />
oils <strong>for</strong> use during the growing season are<br />
available.<br />
Advantages:<br />
1. Economical—most oils are less expensive<br />
than pesticide products.<br />
2. There are no proven cases of insects or<br />
mites becoming resistant to oil. Even<br />
insecticide-resistant species are susceptible<br />
to oil.<br />
3. Oils are environmentally and applicator<br />
friendly, posing little hazard with their<br />
use.<br />
4. When used to control overwintering<br />
pests, growers have more opportunities<br />
to select a day to make an application.<br />
5. Early season feeding damage of overwintering<br />
pests will be eliminated or<br />
signifi cantly reduced.<br />
6. A wide assortment of pests can be<br />
controlled on a large number of hosts.<br />
7. Oil has no residual effect and will only<br />
harm the organism by direct contact.<br />
IPM FOR CHRISTMAS TREE PRODUCTION ........................................................................................................................................................................................................................................... 14
Disadvantages:<br />
1. Oils must come in contact with the<br />
targeted pest to be effective.<br />
2. Field conditions and weather during<br />
applications may limit effi cacy.<br />
3. Eggs will only be controlled if they are<br />
present be<strong>for</strong>e the oil application.<br />
4. Oil has no residual effect.<br />
Cautions:<br />
1. When temperatures are below freezing,<br />
oil can cause phototoxicity. Under<br />
these conditions, the oil and water cannot<br />
stay mixed and the water freezes,<br />
allowing oil droplets to accumulate.<br />
When thawing does occur, the water<br />
will evaporate, concentrating the oil on<br />
plant surfaces.<br />
2. Oil can also cause phototoxicity when<br />
temperatures are above 90°F. Burning is<br />
also a concern on the sunny side of the<br />
trees or during times of drought.<br />
3. Apply oils when conditions allow <strong>for</strong><br />
prompt drying; be sure to avoid drift or<br />
overspray.<br />
4. Mistaken dormancy is a problem in<br />
early fall or late spring. In early fall, if<br />
the plant is not in dormancy and the<br />
leaves are water defi cient, then the oil<br />
will burn the foliage. In late spring, new<br />
growth is burned if the dormant period<br />
ends be<strong>for</strong>e the oil is applied.<br />
5. Conifers known to be sensitive to<br />
dormant oils include Douglas-fi r and<br />
spruce. Be sure not to spray oil on<br />
glaucous, or blue, varieties of conifers<br />
because the blue color will be removed<br />
and may not return <strong>for</strong> 2–3 years.<br />
Genetic variability may affect individual<br />
plants differently, even if the variety<br />
is known to be tolerant to oil.<br />
When using oil, as with any pesticide,<br />
be sure to follow label directions. Some<br />
oils can be mixed with other pesticides to<br />
increase the level of toxicity. Never mix<br />
oils with dimethoate or any type of sulfur,<br />
as serious phytotoxic reactions will result.<br />
Most fungicides are not compatible with<br />
oils. Always consult the oil and insecticide<br />
labels <strong>for</strong> compatibility. Always choose<br />
“superior-type” oils to provide further<br />
insurance that these products are safe.<br />
TRADITIONAL<br />
This largest group of pesticides includes<br />
insecticides, miticides, fungicides, and<br />
herbicides. They can be the most effective<br />
materials available to prevent and destroy<br />
pests; however, they are frequently used<br />
when other options are available. For many<br />
years, broad-spectrum insecticides were the<br />
only tools available to growers. In recent<br />
years, newer, soft materials targeting specifi c<br />
groups of pests have been introduced. These<br />
materials are less likely to disrupt natural<br />
enemies while keeping the target pest in<br />
check.<br />
The use of traditional pesticides should<br />
always be a last resort. If a traditional<br />
pesticide application is a must, choose the<br />
right product, time, and dosage to avoid<br />
problems. Inappropriate use may rid the<br />
area of pests now, but it may also cause a<br />
more serious problem later as pests become<br />
resistant and natural enemies are depleted.<br />
For maximum benefi t and to avoid hazards,<br />
choose <strong>for</strong>mulations that pose the least<br />
threat to nontarget species. Be sure to rotate<br />
classes of pesticides to decrease the chances<br />
of pests developing resistance. Always read<br />
the label be<strong>for</strong>e using any pesticide product.<br />
IPM BASICS ............................................................................................................................................................................................................................................................................................ 15
Step 6. Evaluating Results of<br />
Control Actions<br />
The ultimate goal of any IPM program is<br />
to keep pests under control. Not all control<br />
measures will be met with success. Sudden<br />
rain showers, rapid drop in temperature,<br />
inadequate coverage, and poor mixing are<br />
only a few of the causes of control failures.<br />
In addition, pest populations may rebound<br />
quickly despite the best techniques of an<br />
applicator. Monitoring pests after a control<br />
application is equally as important as<br />
monitoring them be<strong>for</strong>e the application.<br />
Scouts should check the target pests 3–5<br />
days after any application. Some materials<br />
may require a longer time period to be effective.<br />
Checking effectiveness of fungicides is<br />
very diffi cult, but trees should be examined<br />
to make sure a spray residue is on all areas of<br />
the tree. Heed reentry interval requirements<br />
on the pesticide label when scouting after<br />
an application.<br />
If the target pest is still present in suffi<br />
cient numbers to cause damage, a repeat<br />
application may be necessary. Consider the<br />
following factors when determining the<br />
need <strong>for</strong> reapplication of pesticides:<br />
• <strong>Pest</strong> life cycle:<br />
— Vulnerability of certain life stages<br />
— Timing of generations<br />
— Number of generations per season<br />
• <strong>Pest</strong>icide properties:<br />
— Mode of action<br />
— Residual effect<br />
• Weather conditions following the<br />
application<br />
• Number of applications permitted<br />
according to the pesticide label<br />
Even the best method of control will not<br />
eliminate the need <strong>for</strong> continued monitoring<br />
of pests. Many pests are capable of<br />
reappearing at a later date. They may be<br />
blown in from neighboring fi elds or woodlots,<br />
arrive with new seedlings, or have been<br />
present in an undetectable level during<br />
regular scouting.<br />
Pennsylvania Conifer Certifi cation<br />
and Quarantine Guidelines<br />
Note: Growers in other states should consult<br />
with their regulatory authority <strong>for</strong> their state<br />
quarantine guidelines.<br />
Conifer/<strong>Christmas</strong> tree growers who plan<br />
to sell B&B trees, seedlings, and transplants<br />
must have a Pennsylvania state nursery<br />
certifi cate. Pennsylvania’s Plant <strong>Pest</strong> Act<br />
requires that all nursery stock (i.e., plants<br />
with roots) must be inspected and certifi ed<br />
as apparently free of injurious plant pests<br />
be<strong>for</strong>e being removed from the growing site.<br />
To obtain a nursery certifi cate, contact the<br />
Pennsylvania Department of Agriculture<br />
Bureau of Plant Industry Regional Offi ce<br />
nearest you to request an application.<br />
The inspector assigned to your county will<br />
inspect your trees, give recommendations<br />
<strong>for</strong> controls of any pest or disease problems<br />
found, and certify trees suitable <strong>for</strong> digging<br />
as nursery stock. This applies to retail or<br />
wholesale, including local sales.<br />
Any conifer grower who ships trees<br />
across state lines should be aware of federal<br />
and state quarantines that may apply to cut<br />
and B&B trees, seedlings, and transplants.<br />
Federal quarantines include the gypsy<br />
moth quarantine. Under this quarantine,<br />
all cut and B&B trees of any species moving<br />
from quarantined areas into or through<br />
nonquarantined areas must be inspected and<br />
certifi ed free of all gypsy moth life stages.<br />
Movement of trees within the quarantined<br />
area does not require gypsy moth certifi cation.<br />
Maps of the quarantined areas may<br />
be found on the USDA Web site at www<br />
.aphis.usda.gov.<br />
Pine shoot beetle is another federal<br />
quarantine that affects the movement of all<br />
Pinus species—eastern white, Scotch, Austrian,<br />
and so on. All cut and B&B pine trees<br />
or any parts, such as branches, wreaths, etc.,<br />
moving from a quarantined to a nonquarantined<br />
area must be inspected and certifi ed<br />
free of pine shoot beetle. Maps of the pine<br />
shoot beetle quarantine zone may also be<br />
found at www.aphis.usda.gov.<br />
IPM FOR CHRISTMAS TREE PRODUCTION ........................................................................................................................................................................................................................................... 16
Each individual state of the United<br />
States also has its own list of quarantine<br />
pests. Some examples include the following:<br />
1. Cereal leaf beetle in Cali<strong>for</strong>nia—movement<br />
of Scotch, red, and Austrian pine<br />
trees from Pennsylvania to Cali<strong>for</strong>nia is<br />
regulated.<br />
2. European pine shoot moth in Cali<strong>for</strong>nia,<br />
Hawaii, Idaho, Montana, Nevada,<br />
and Oregon is regulated.<br />
3. Hemlock woolly adelgid in Maine,<br />
Michigan, New Hampshire, Ohio, Vermont,<br />
and Wisconsin—some of these<br />
states do not allow any hemlock to be<br />
shipped there, while others will allow<br />
them if they meet guidelines.<br />
4. Japanese beetle—there are four categories<br />
of states based on whether they are<br />
considered infested or noninfested:<br />
• Category 1, Uninfested/Quarantine<br />
<strong>Pest</strong>: Japanese beetle is not known to<br />
occur and is considered a high/moderate-risk<br />
pest. These states consist<br />
mainly of the western United States<br />
and require quarantine certifi cation<br />
in order to ship nursery stock to them<br />
from Pennsylvania.<br />
• Category 2, Uninfested or Partially<br />
Infested/Regulated Nonquarantine<br />
<strong>Pest</strong>: Japanese beetle is not known<br />
to occur but is established in limited<br />
areas of the state and is likely to<br />
spread. The impact can be mitigated<br />
to an acceptable level by applying<br />
a treatment be<strong>for</strong>e shipping. These<br />
states consist mainly of the midwestern<br />
United States.<br />
• Category 3, Generally Infested and<br />
Partially Infested: This includes<br />
states where infestations are generally<br />
widespread. Since Pennsylvania is<br />
considered Category 3 (infested), regulations<br />
<strong>for</strong> midwestern and western<br />
states require that a special inspection<br />
be per<strong>for</strong>med or a chemical treatment<br />
option <strong>for</strong> Japanese beetle grubs be<br />
per<strong>for</strong>med be<strong>for</strong>e B&B trees, transplants,<br />
or seedlings may be shipped<br />
there. The USDA has enacted a<br />
Japanese Beetle Harmonization Plan<br />
designed to slow the artifi cial movement<br />
of Japanese beetles from the<br />
eastern United States to the midwestern<br />
and western states. This plan<br />
specifi es each state’s requirements <strong>for</strong><br />
shipments, which must be documented<br />
by a plant inspector be<strong>for</strong>e<br />
shipment may be sent. Generally, the<br />
East Coast states are in this category.<br />
• Category 4, Historically Not Known<br />
to Be Infested/No Regulatory Signifi -<br />
cance: Japanese beetle spread is not<br />
likely or the insect is not likely to<br />
survive or become a pest. The states<br />
of Florida and Colorado fall into this<br />
category.<br />
5. Bagworm in Delaware—the state of<br />
Delaware includes cut <strong>Christmas</strong> trees<br />
in their defi nition of nursery stock. Any<br />
cut or B&B trees must be inspected and<br />
found free of bagworm and gypsy moth<br />
in particular be<strong>for</strong>e any shipments are<br />
made to Delaware.<br />
The regulations <strong>for</strong> federal quarantines<br />
and each individual state’s quarantines may<br />
be found on the Web at nationalplantboard.org.<br />
State inspectors and the plant<br />
protection specialist from the Pennsylvania<br />
Department of Agriculture can provide<br />
in<strong>for</strong>mation about these quarantines.<br />
IPM BASICS ............................................................................................................................................................................................................................................................................................ 17
This chart provides a list of growing degree<br />
day ranges (see page 9 <strong>for</strong> explanation) that<br />
correspond with a particular pest life stage<br />
or life cycle event that is critical in the control<br />
of that pest. This in<strong>for</strong>mation combined<br />
with scouting observations will help growers<br />
achieve the best control of pest problems on<br />
their farms.<br />
Growing Degree Day Ranges <strong>for</strong> <strong>Christmas</strong> <strong>Tree</strong> <strong>Pest</strong>s<br />
<strong>Pest</strong> Name Degree Day Range Life Stage Comments<br />
Bagworm 650–750* Larvae emerge from bags<br />
Balsam twig aphid 30–100 Overwintering eggs hatch<br />
Conifer rust mites (eriophyid) 7–22 Overwintering eggs hatch<br />
Cooley spruce gall adelgid 22–81 Spring control of overwintering stage<br />
2,800–3,000 Fall control of overwintering stage<br />
Cryptomeria scale 600–800* First-generation crawlers emerge<br />
1,750–2,130* Second-generation crawlers emerge<br />
Douglas-fi r needle midge 200–400* Adults emerge from soil<br />
Eastern pine weevil 7–100 Overwintering adults become active<br />
Eastern spruce gall adelgid 22–170 Spring control of overwintering stage<br />
2,800–3,000 Fall control of overwintering stage<br />
Elongate hemlock scale 360–700 Crawlers fi rst become active<br />
European pine sawfl y 78–220 Overwintering eggs hatch; larvae present<br />
Gypsy moth 90–448 Overwintering eggs hatch; larvae present<br />
Introduced pine sawfl y 400–600 Overwintering eggs hatch; larvae present<br />
Pales weevil 7–121 Overwintering adults become active;<br />
treatment to prevent egg laying<br />
Pine bark adelgid 22–58 Spring control of overwintering stage<br />
Pine needle scale 298–448 First-generation crawlers emerge<br />
1,290–1,917 Second-generation crawlers emerge<br />
Pine root collar weevil 300–350 Overwintering adults become active<br />
Pine shoot beetle 450–550 New adults emerge<br />
Redheaded pine sawfl y 400–600 Overwintering eggs hatch; larvae present<br />
Spruce spider mite 50–121* Overwintering eggs hatch<br />
Striped pine scale 400–500 Eggs hatch and fi rst crawlers emerge<br />
(Toumeyella sp.)<br />
White pine weevil 7–58 Overwintering adults become active<br />
Zimmerman pine moth 121–246 Larvae emerge from overwintering sites<br />
*Based on observations in Pennsylvania.<br />
Monitoring of GDDs begins March 1.<br />
IPM FOR CHRISTMAS TREE PRODUCTION ........................................................................................................................................................................................................................................... 18
This calendar gives a range of weeks of<br />
occurrence <strong>for</strong> each pest and pest event.<br />
The purpose of this calendar is to show<br />
growers how dates of particular pest events<br />
relate to others throughout the growing<br />
season. Because only weeks are given rather<br />
than specifi c dates, these are just approximate<br />
ranges. The in<strong>for</strong>mation in this calendar<br />
was collected from 10 years of scouting<br />
Insect and Mite Calendar<br />
<strong>Pest</strong> Event<br />
Bagworm larvae emerge<br />
Balsam twig aphid eggs hatch<br />
Cooley spruce gall adelgid waxing over<br />
Cryptomeria scale crawlers emerge<br />
Douglas-fi r needle midge adults emerge<br />
Eastern spruce gall<br />
adelgid<br />
waxing over<br />
Eastern pine weevil adults emerge<br />
Elongate hemlock scale crawlers emerge<br />
Eriophyid mite* eggs hatch<br />
Gypsy moth larvae emerge<br />
Pales weevil adults emerge<br />
Pine bark adelgid crawlers emerge<br />
Pine needle scale crawlers emerge<br />
Sawfl ies larvae emerge<br />
Spruce spider mite* eggs hatch<br />
Striped pine scale crawlers emerge<br />
White pine weevil adults emerge<br />
*Population resurgence in September may require treatment, if numbers are high.<br />
early/late emergence<br />
peak emergence<br />
data in south-central and southeastern<br />
Pennsylvania, so these ranges will differ<br />
from the actual calendar dates in other areas<br />
of the country. This in<strong>for</strong>mation should be<br />
combined with growing degree day calculations<br />
and scouting data to determine local<br />
occurrence. Bud break occurs from April to<br />
May and signals emergence of various insects,<br />
as well as infection periods of disease.<br />
Growing Season (weeks)<br />
March April May June July August<br />
1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4<br />
IPM BASICS ............................................................................................................................................................................................................................................................................................ 19
PEST FACT SHEETS<br />
In<strong>for</strong>mation on disease, insect, and mite<br />
pests that affect <strong>Christmas</strong> trees in Pennsylvania<br />
is presented in the following 40<br />
pest fact sheets. The list of pests is not<br />
all inclusive. <strong>Pest</strong>s that cause occasional<br />
or minimal damage or pests that may be<br />
introduced after printing of the manual<br />
are not included at this time but may be<br />
added at a later date. If you are using this<br />
manual outside of Pennsylvania, you may<br />
encounter pests that are not included because<br />
they do not occur in our state. The<br />
pests have been divided into three categories<br />
based on the type of injury they cause:<br />
Needle Discoloration and Injury (pages<br />
22–71), Shoot and Branch Injury (pages<br />
72–104), and Stem and Root Injury/<strong>Tree</strong><br />
Mortality (pages 106–121).
NEEDLE DISCOLORATION<br />
AND INJURY<br />
The pest fact sheets on pages 23–71 are<br />
<strong>for</strong> disease, insect, and mite pests that<br />
damage needles and result in defoliation,<br />
discoloration, or distortion. The damage<br />
can vary from slight yellowing of needles<br />
to total loss of needles. <strong>Tree</strong> death is uncommon<br />
and only occurs if a pest population<br />
is unmanaged <strong>for</strong> several seasons.<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 22
Hosts<br />
• All species of <strong>Christmas</strong> trees and<br />
ornamental conifers<br />
• Occasionally found on deciduous trees<br />
and shrubs<br />
• Host list includes more than 120 species<br />
of trees and shrubs<br />
Damage Potential<br />
• Moderate–high<br />
Symptoms and Signs<br />
• Brown spots on foliage<br />
• Missing needles (current year’s growth)<br />
• Brown, conelike bags hanging from<br />
branches<br />
• Dead branches<br />
Causes of Similar Symptoms<br />
• Gypsy moths occasionally defoliate some<br />
<strong>Christmas</strong> trees<br />
• Grass bagworm and snail-cased bagworm<br />
occasionally found on conifers but do not<br />
cause damage<br />
Identifi cation<br />
Bagworm is a caterpillar that molts into a<br />
moth in the adult stage. They are easiest to<br />
identify by the bags they construct as they<br />
feed. Bags on spruce will look completely<br />
different from those on arborvitae or honey<br />
locust because the host plant material is<br />
incorporated into the bag. Each bag can be<br />
up to 1½–2½ inches (38–63 millimeters,<br />
mm) long when the larva is mature. If you<br />
observe a bag closely, you will see that the<br />
caterpillar’s shiny black head and fi rst pair of<br />
legs are exposed as it feeds. The robust body<br />
of the caterpillar is pale yellow, mottled<br />
with black, and may be up to 1½ inches<br />
(38 mm) long when mature.<br />
Adult bagworms are seldom seen.<br />
Females have no wings and never emerge<br />
from the bag they construct in the caterpillar<br />
stage. Since they do not have wings<br />
or legs, they resemble maggots. The male<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
moths have two pairs of wings and three<br />
pairs of legs. Their wings are transparent<br />
with black borders and their bodies are dark<br />
and fuzzy. Male bagworms are about ¾ inch<br />
(15–18 mm) long from head to the tip of<br />
the abdomen. Their wingspan is approximately<br />
1 inch (25 mm).<br />
Biology and Life Cycle<br />
Bagworms overwinter as eggs inside the bag<br />
constructed by the female (Fig. 1). In late<br />
May through mid-June, eggs hatch and the<br />
larvae crawl out the bottom of this bag.<br />
They spin down on a thin strand of silk (a<br />
habit known as “ballooning”). Larvae will<br />
settle to feed on lower branches or may be<br />
blown to nearby plants during the ballooning<br />
stage. When they reach a suitable host,<br />
the larvae begin to feed and produce silk<br />
to construct individual bags around their<br />
bodies (Fig. 2). Plant debris is woven into<br />
Figure 1. Bagworm eggs inside the female’s<br />
bag. Courtesy of Sandy Gardosik, PDA<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
Monitor in late August–September <strong>for</strong> male moth emergence (see Control Options section).<br />
Figure 2. Immature<br />
larvae feeding<br />
on Douglas-fi r;<br />
needles show<br />
symptoms of early<br />
damage. Courtesy<br />
of Sandy Gardosik,<br />
PDA<br />
BAGWORM<br />
Thyridopteryx<br />
ephemerae<strong>for</strong>mis<br />
(Haworth)<br />
Bagworm feeding damage.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
BAGWORM ............................................................................................................................................................................................................................................................................................ ..................................................................... 24 23
Figure 3. Immature bagworm<br />
larva. Courtesy of Sandy<br />
Gardosik, PDA<br />
Figure 4. Bagworm larva inside<br />
open bag. Courtesy of Pennsylvania<br />
DCNR Forestry Archive,<br />
Bugwood.org (#5020082)<br />
Figure 5. Heavy bagworm<br />
infestation on Colorado blue<br />
spruce. Courtesy of Rayanne D.<br />
Lehman, PDA<br />
Figure 6. Bagworm larval bag<br />
attached with silk to eastern<br />
white pine. Courtesy of Cathy<br />
Thomas, PDA<br />
each bag to camoufl age and protect the<br />
larva. The larva feeds through an opening at<br />
the top of the bag, while the pointed end of<br />
the bag dangles from the host. Very young<br />
larvae may carry their bags in a snail-like<br />
manner <strong>for</strong> a short time (Fig. 3).<br />
Only the newly hatched larva and the<br />
male moth can be found outside a protective<br />
bag. As the larva grows, it enlarges the<br />
bag by adding more silk and plant material<br />
(Fig. 4). The bag is attached to branches<br />
of the host with loose strands of silk and<br />
can be moved to get to fresh plant material<br />
(Fig. 5). After feeding <strong>for</strong> 8–10 weeks, the<br />
larva fi rmly attaches the bag to a branch<br />
with stronger, more durable silk and pupates<br />
inside the bag (Fig. 6). About 4 weeks later,<br />
the adult male bagworm emerges.<br />
After a male moth (Fig. 7) emerges<br />
through the bottom of the bag, the pupal<br />
case can occasionally be seen partially<br />
protruding from the empty bag (Fig. 8). The<br />
female never leaves her bag but positions<br />
herself over a small opening in the bottom<br />
of the bag and begins to emit sex pheromones<br />
to attract male moths. Mating occurs<br />
with the male outside the bag and female<br />
inside. Adults do not feed and die shortly<br />
after mating. Each female can produce<br />
300–1,000 eggs be<strong>for</strong>e dying. The eggs will<br />
remain inside the female’s mummifi ed body,<br />
inside her bag, through the winter. If you<br />
squeeze a bag containing eggs during the<br />
winter months, the small, whitish eggs will<br />
ooze out and may resemble minute tapioca.<br />
Only one generation of bagworm occurs per<br />
year in Pennsylvania.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• No recommendations are available<br />
at this time.<br />
Preseason<br />
• Be<strong>for</strong>e new growth starts, scout trees <strong>for</strong><br />
brown bags; hand-pick and destroy bags.<br />
Remove tough silk around the branch to<br />
prevent girdling as the branch grows.<br />
• Be<strong>for</strong>e mid-May, tag at least one tree<br />
infested with bagworm to monitor <strong>for</strong><br />
emergence of larvae.<br />
Figure 7. Adult male bagworm adhered to a<br />
pheromone-baited sticky trap. Courtesy of<br />
Tracey Olson, PDA<br />
Figure 8. Pupal<br />
case exposed after<br />
male bagworm<br />
emergence.<br />
Courtesy of Sandy<br />
Gardosik, PDA<br />
Growing Season<br />
• Threshold level: At this time, no threshold<br />
level has been established, but infestations<br />
on spruce can easily cause economic<br />
damage.<br />
• Scout <strong>for</strong> emerging young larvae on<br />
lower branches in late May to early June.<br />
Typically, emerging larvae drop down from<br />
bags to begin feeding and gradually move<br />
to the top of the plant as they mature.<br />
• Growing degree days: Based on observations<br />
in Pennsylvania, larvae emerge at<br />
650–750 GDDs.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 24
Control Options<br />
Biological<br />
• Bagworm has several naturally occurring<br />
insect predators (wasps and hornets)<br />
and parasitoids (wasps and fl ies) (Fig. 9),<br />
fungal parasites, and bird predators. No<br />
species are available <strong>for</strong> augmentation at<br />
this time.<br />
Mechanical<br />
• Hand-picking and destroying bags anytime<br />
during growing season or in spring<br />
be<strong>for</strong>e eggs hatch can be very effective in<br />
eliminating a localized infestation.<br />
• Remove and burn or fi nely chip severely<br />
infested trees within and around the<br />
plantation.<br />
Biorational<br />
• Bacillus thuringiensis (Bt) is a bacterium<br />
that kills specifi c insects, such as caterpillars,<br />
but is safer <strong>for</strong> natural enemies. It is<br />
most effective when used against young<br />
larvae. Larvae must consume Bt on the<br />
plant material; there<strong>for</strong>e, coverage and<br />
timing are critical.<br />
• Pheromone traps can be deployed in<br />
August to trap male moths and decrease<br />
the number of mated females (Fig. 10).<br />
These traps contain a chemical that attracts<br />
males by mimicking the female sex<br />
pheromone. When males are trapped,<br />
fewer matings occur and the number of<br />
overwintering eggs will decrease.<br />
Chemical<br />
• Insecticides are most effective when<br />
applied to young larvae. After fi rst treatment,<br />
monitor populations to determine<br />
if second treatment is warranted.<br />
Next Crop/Prevention<br />
• Inspect new seedlings <strong>for</strong> signs of bags;<br />
remove bags.<br />
Figure 9. Parasitoid wasp (circled) inserting her<br />
eggs into the bagworm larval case. Courtesy of<br />
Sarah Pickel, PDA<br />
Figure 10. Bagworm pheromone-baited sticky<br />
trap. Courtesy of Sarah Pickel, PDA<br />
BAGWORM ............................................................................................................................................................................................................................................................................................. 25
BALSAM TWIG<br />
APHID<br />
Mindarus abietinus<br />
Koch<br />
Twisted needle symptom.<br />
Courtesy of Tracey Olson, PDA<br />
Hosts<br />
• All species of true fi r, especially Fraser and<br />
balsam<br />
• Rarely found on some spruce and<br />
juniper<br />
Damage Potential<br />
• Moderate–high<br />
Symptoms and Signs<br />
• Curled, twisted needles on current year’s<br />
growth<br />
• Stunted needles<br />
• Black sooty mold and presence of stinging<br />
insects<br />
Causes of Similar Symptoms<br />
• <strong>Pest</strong>icide use<br />
• Soft scales<br />
• Other aphids<br />
Identifi cation<br />
Balsam twig aphids are tiny, soft-bodied<br />
insects with piercing-sucking mouthparts.<br />
Most stages are pale bluish green and some<br />
may have powdery, wax strands adhering to<br />
the body. The second generation is the only<br />
one that produces winged <strong>for</strong>ms; all other<br />
generations have only wingless adults. The<br />
largest stage, the “stem mother,” occurs in<br />
the fi rst generation and at maturity is 1 ⁄25– 1 ⁄13<br />
inch (1–2 mm) long.<br />
Eggs are small ovals coated with waxy<br />
rods that have sloughed off from the<br />
underside of the female. Initially, eggs are<br />
pale tan, but they darken with age and by<br />
spring appear to be silvery black. With the<br />
white, waxy rods and almost black eggs, it is<br />
relatively easy to spot eggs using a hand lens<br />
of at least 15X magnifi cation.<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Biology and Life Cycle<br />
The balsam twig aphid has a complex life<br />
cycle with three to four generations occurring<br />
per year. Most of the year is spent in<br />
the silvery egg stage on twigs of the host tree<br />
(Fig. 1). In early April, prior to bud break,<br />
the overwintering egg hatches into a small<br />
nymph (Fig. 2). This nymph feeds <strong>for</strong> a<br />
period of time on the underside of last year’s<br />
needles be<strong>for</strong>e molting into the wingless<br />
stem mother. As buds are swelling and just<br />
beginning to open, the stem mother moves<br />
Figure 1. Silvery balsam twig aphid egg at<br />
the needle’s base on the underside of a twig.<br />
Courtesy of Rayanne D. Lehman, PDA<br />
Figure 2. Newly hatched nymph feeding on<br />
needles and secreting “honeydew.” Courtesy of<br />
Rayanne D. Lehman, PDA<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
NEEDLE DISCOLORATION AND INJURY .......... ................................................................................................................................................................................................................................................ 26
to the bud (Fig. 3) and gives live birth to<br />
up to 70 second-generation nymphs. No<br />
mating or eggs are involved; the nymphs are<br />
clones of the stem mother and capable of<br />
feeding immediately.<br />
Figure 3. Stem mother on needle bud. Courtesy<br />
of Rayanne D. Lehman, PDA<br />
This second generation is the most<br />
damaging (Fig. 4). Perfectly synchronized<br />
with bud break, the nymphs begin to feed<br />
on the elongating needles (Fig. 5), causing<br />
distortion and stunting (Fig. 6). Within a<br />
short time, they have matured and eventually<br />
produce live, either wingless or winged<br />
females. If wingless <strong>for</strong>ms are produced, they<br />
continue to feed on the same tree. Although<br />
the winged females are weak fl iers, they are<br />
capable of short fl ights or becoming airborne<br />
to be carried by air currents to new hosts.<br />
When the winged females reach the<br />
new host or new stem on same tree, they<br />
produce both wingless females and males<br />
(Fig. 7). This last generation is the only<br />
one with males that mate with the females.<br />
Mated females deposit one or two overwintering<br />
eggs close to the buds be<strong>for</strong>e dying.<br />
This is also the only time in the life cycle<br />
that eggs are produced.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Avoid planting at lower elevations and on<br />
slopes where aphid populations tend to be<br />
higher.<br />
• Plant species that have delayed bud break.<br />
Balsam fi r tends to break bud early and is<br />
most severely infested; Fraser is next to<br />
break bud; Canaan generally breaks bud<br />
last.<br />
• Do not buy infested trees or nursery stock.<br />
Preseason<br />
• Between July and March, assess damage<br />
levels from the previous year’s infestation.<br />
It is most important to control this pest<br />
during the last 2 years be<strong>for</strong>e harvest in<br />
order to produce 2 years of good, straight<br />
growth. On young trees, excessive damage<br />
will stunt overall growth.<br />
• Do not apply a nitrogen fertilizer be<strong>for</strong>e<br />
bud break to a previously infested area.<br />
This tends to increase aphid populations.<br />
• Look <strong>for</strong> overwintering eggs and tag trees<br />
to monitor <strong>for</strong> egg hatch by the end of<br />
March.<br />
• Growing degree days: Egg hatch occurs at<br />
30–100 GDDs.<br />
• Begin monitoring <strong>for</strong> egg hatch by April<br />
1 in southern counties. Using a hand<br />
lens, examine the underside of last year’s<br />
needles <strong>for</strong> single, blue-green nymphs.<br />
These nymphs are usually within 2 inches<br />
of the bud and often have a large droplet<br />
of clear honeydew on their tail end.<br />
• Encourage natural predators and parasitoids.<br />
Growing Season<br />
• Scout <strong>for</strong> aphids by beating 10 inches of<br />
foliage over a piece of dark paper. Select<br />
15 trees per acre (similar age, size, and<br />
location) and sample two sides of each<br />
tree. Count aphids and predators found. In<br />
April or early May, it may be necessary to<br />
apply chemical control if more than three<br />
aphids per tree are found.<br />
• As bud break begins, look at the opening<br />
buds to fi nd the stem mothers and secondgeneration<br />
nymphs.<br />
• When vegetative cones are produced on<br />
trees, break open the cones and look <strong>for</strong><br />
nymphs that have migrated here to feed.<br />
• Observe presence of lady beetles since<br />
several species are important predators.<br />
• Observe presence of stinging insects as<br />
new growth elongates. The bees and wasps<br />
are attracted to the honeydew produced<br />
by the feeding aphids.<br />
• At the end of the season, update records<br />
and evaluate results.<br />
Figure 4. Balsam<br />
twig aphid nymphs.<br />
Courtesy of<br />
Rayanne D. Lehman,<br />
PDA<br />
Figure 5. Nymphs (circled) feeding<br />
in a barely opened bud.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
Figure 6. Needle twisting or<br />
kinking evident of balsam twig<br />
aphid damage. Courtesy of<br />
Cathy Thomas, PDA<br />
Figure 7. Winged balsam twig<br />
aphid female. Courtesy of<br />
Rayanne D. Lehman, PDA<br />
BALSAM TWIG APHID ............................................................................................................................................................................................................................................................................ 27
Figure 8. Larva of multicolored<br />
Asian lady beetle. Courtesy of<br />
Cathy Thomas, PDA<br />
Control Options<br />
Biological<br />
• Balsam twig aphids have numerous<br />
naturally occurring predators, including<br />
yellow jackets, lacewings, earwigs, lady<br />
beetles and their larvae (Fig. 8), assassin<br />
bugs, ants, big-eyed bugs, predatory thrips,<br />
hover fl y larvae, syrphid fl ies, and predaceous<br />
midges. The introduced multicolored<br />
Asian lady beetle is an excellent<br />
aphid predator in the adult and larval<br />
stage. Parasitoids of balsam twig aphids are<br />
Aphidius wasps. Refer to Appendix B: Biological<br />
Controls Photo Chart <strong>for</strong> pictures.<br />
Mechanical<br />
• Pick and destroy young cones in spring<br />
during aphid activity. Since pesticides will<br />
not reach the aphids inside cones, this<br />
practice can be time consuming but very<br />
benefi cial and will positively affect the<br />
aesthetics of the tree.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• All pesticide intervention should occur<br />
after egg hatch but be<strong>for</strong>e bud break;<br />
after bud break, aphids are protected by<br />
new growth and some damage has already<br />
occurred. Dormant oil will kill aphids but<br />
will have limited effect on overwintering<br />
eggs and often causes damage to elongating<br />
needles.<br />
Next Crop/Prevention<br />
• Purchase and plant pest-free nursery stock<br />
from a reputable company.<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 28
Hosts<br />
• Douglas-fi r<br />
• Alternate hosts: Colorado blue spruce and<br />
occasionally other spruces (see Shoot and<br />
Branch Injury section <strong>for</strong> Cooley spruce<br />
gall adelgid on spruce)<br />
Damage Potential<br />
• Moderate<br />
Symptoms and Signs<br />
• Yellow spots on the needles<br />
• Needles with bends or crooks<br />
• Small, white, cottony balls on the<br />
underside of needles<br />
• Premature needle drop<br />
Causes of Similar Symptoms<br />
• Rhabdocline needle cast<br />
• Douglas-fi r needle midge<br />
Identifi cation<br />
The Cooley adelgid is a small, soft-bodied<br />
insect with sucking mouthparts. Both<br />
winged and wingless <strong>for</strong>ms exist, but the<br />
winged insect is not often seen. The easiest<br />
method of identifi cation is the characteristic<br />
damage. On Douglas-fi r, Cooley adelgid<br />
does not cause galls, as it does on spruce.<br />
Instead, it causes pale or yellow spots on the<br />
top of the needle. Frequently, the needle<br />
will have a bend or crook at this site. These<br />
spots or bends indicate feeding sites <strong>for</strong> the<br />
nymphs, which will be found on the underside<br />
of the needle. The crawlers and early<br />
nymphs are dark brown to black, fl at, and<br />
have ridges across their bodies. They can<br />
be seen using a hand lens with at least 15X<br />
magnifi cation. White, cottony masses may<br />
also be found on the underside of needles at<br />
certain times of year.<br />
In early spring, examine the underside of<br />
needles on Douglas-fi r. The egg masses will<br />
be beneath a waxy mass on needles of outer<br />
growth. At bud break, look at the base of<br />
elongating shoots <strong>for</strong> minute, dark nymphs<br />
feeding on the new needles.<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Biology and Life Cycle<br />
The life cycle of Cooley adelgid is<br />
complex and involves distinctly different<br />
<strong>for</strong>ms on Colorado spruce and Douglasfi<br />
r. The pest can alternate between the<br />
two hosts or produce continual generations<br />
on either. Both winged and<br />
wingless <strong>for</strong>ms are found; all adults on<br />
Douglas-fi r are female.<br />
On Douglas-fi r, exposed nymphs<br />
overwinter on the underside of the<br />
needles (Fig. 1). In early spring, these<br />
nymphs begin to feed and produce<br />
their waxy protective threads. Initially,<br />
the white wax is just a fringe around<br />
the dark body (Fig. 2), but by the time<br />
they reach maturity, the entire body is<br />
engulfed (Figs. 3 and 4).<br />
Figure 1.<br />
Overwintering<br />
nymphs. Courtesy<br />
of Sandy Gardosik,<br />
PDA<br />
Figure 2. Nymph<br />
with waxy fringe<br />
beginning to <strong>for</strong>m<br />
around the body.<br />
Courtesy of Sandy<br />
Gardosik, PDA<br />
Figure 3. Nymph<br />
covered with white,<br />
waxy fringe.<br />
Courtesy of Sandy<br />
Gardosik, PDA<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
Spray must occur be<strong>for</strong>e nymphs wax over.<br />
Figure 4. Nymphs<br />
covered with white,<br />
waxy fringe.<br />
Courtesy of Brian<br />
Schildt, PDA<br />
COOLEY<br />
SPRUCE GALL<br />
ADELGID ON<br />
DOUGLAS-FIR<br />
Adelges cooleyi<br />
(Gillette)<br />
White, waxy tufting of<br />
Cooley spruce gall adelgid.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
COOLEY SPRUCE GALL ADELGID ON DOUGLAS-FIR ........................................................................................................................................................................................................................... ..................................................................... 29
Figure 5. Cooley eggs laid<br />
under the waxy fringe of the<br />
mature female. Courtesy of<br />
Sandy Gardosik, PDA<br />
Figure 6. Cooley nymphs<br />
feeding on new growth of<br />
Douglas-fi r, resulting in needle<br />
kinking. Courtesy of PDA<br />
Figure 7. Kinked needles and<br />
chlorosis caused by nymph<br />
feeding. Courtesy of<br />
Rayanne D. Lehman, PDA<br />
Two types of females result from these<br />
overwintering <strong>for</strong>ms: wingless females that<br />
will remain on Douglas-fi r and winged<br />
females that can fl y to spruce and complete<br />
a nondamaging generation. The wingless<br />
females produce a cluster of up to 100 eggs<br />
(Fig. 5), which begin to hatch as trees break<br />
bud. Nymphs crawl into the opening buds<br />
and feed on the elongating needles (Fig. 6).<br />
Chlorotic spots and bending of the needles<br />
result from the nymphs feeding, but no gall<br />
results. (Fig. 7). Subsequent generations<br />
occur on Douglas-fi r through the season, but<br />
the fi rst generation is the most damaging.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• When planting, separate Colorado blue<br />
spruce and Douglas-fi r trees. This will not<br />
eliminate problems, but it may help lessen<br />
the severity.<br />
• Plant resistant/tolerant Douglas-fi r<br />
varieties.<br />
• Remove any mature Colorado blue spruce<br />
or Douglas-fi r that may be sources of<br />
infestation.<br />
Preseason<br />
• Scout <strong>for</strong> overwintering nymphs on the<br />
underside of needles.<br />
• Examine the undersides of needles on<br />
inner branches as well as last year’s<br />
growth.<br />
Growing Season<br />
• Growing degree days: The recommended<br />
control period against nymphs is 22–81<br />
GDDs in the spring (be<strong>for</strong>e nymphs wax<br />
over) and 2,800–3,000 GDDs in the fall.<br />
• Threshold level: No threshold has been<br />
established. Control <strong>for</strong> 2 years be<strong>for</strong>e<br />
harvest to have damage-free needles.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• Encourage natural predators such as<br />
lacewings, assassin bugs, and lady beetles.<br />
Mechanical<br />
• No recommendations are available<br />
at this time.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Dormant oil: Overwintering nymphs can<br />
be controlled by applying dormant oil<br />
be<strong>for</strong>e new growth starts in early spring or<br />
in late fall after fi rst frost when trees are<br />
not actively growing.<br />
• Spring insecticide: The fi rst application<br />
should occur after nymphs/immature<br />
females begin to swell but be<strong>for</strong>e they<br />
produce white, waxy threads to cover<br />
themselves. A second application in 7–10<br />
days may be needed but must occur be<strong>for</strong>e<br />
bud break.<br />
• Fall insecticide: A single spray should be<br />
applied in late September or October to<br />
control the exposed nymphs and immature<br />
females be<strong>for</strong>e overwintering.<br />
• Insecticide applications are not recommended<br />
during the growing season since<br />
most stages will be protected under waxy<br />
threads or inside galls.<br />
Next Crop/Prevention<br />
• Purchase and plant pest-free nursery stock<br />
from a reputable company.<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 30
Hosts<br />
• True fi rs, especially Fraser, Canaan, and<br />
balsam<br />
• All other conifer species<br />
Damage Potential<br />
• Moderate–high<br />
Symptoms and Signs<br />
• Damage to denser, lower, and inner<br />
branches of the tree<br />
• Mottled yellowing on needle tops<br />
• Premature needle drop<br />
Causes of Similar Symptoms<br />
• Elongate hemlock scale<br />
• Spruce spider mite<br />
Identifi cation<br />
Cryptomeria scale is an armored scale found<br />
on the underside of needles. Observed with<br />
a hand lens, the scale resembles a fried<br />
egg. The yellow, soft-bodied scale insect is<br />
protected beneath a whitish-gray, oval cover.<br />
Yellow, cast skins of the immature scales can<br />
be seen centrally under the translucent scale<br />
cover. The scale covering is the structure<br />
most frequently observed and will easily<br />
slough off when rubbed with your fi nger.<br />
When the scale covering is removed, it<br />
frequently leaves a solid green ring on the<br />
white stomata on the needle. The female<br />
covering is about 1 ⁄20 inch (1.0–1.5 mm)<br />
long and the immature male covering is<br />
slightly smaller. Underneath the covering,<br />
the adult female scale is a yellow, fl attened<br />
oval that lacks legs or a distinct head. Prying<br />
the insect from the needle may reveal the<br />
extremely long, thin mouthparts inserted<br />
in the needle. Adult male scales are winged<br />
and rarely seen. Eggs deposited under the<br />
female covering are oval and yellow. The<br />
pale yellow, lozenge-shaped crawlers are<br />
about 1 ⁄100 inch long (0.25 mm) and visible<br />
to the naked eye after they have moved from<br />
under the female cover.<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Biology and Life Cycle<br />
All stages of Cryptomeria scale are found on<br />
the underside of needles. The crawlers and<br />
males are the only stages capable of moving<br />
about on the needles. All other immature<br />
stages and the females are sedentary, remaining<br />
on the site where they fi rst inserted their<br />
piercing-sucking mouthparts to feed (Fig. 1).<br />
This feeding causes chlorotic spotting on the<br />
needles (Fig. 2), which may give an infested<br />
tree a yellow appearance beginning at its<br />
base (Fig. 3).<br />
Figure 1.<br />
Heavy<br />
infestation<br />
of mature<br />
Cryptomeria<br />
scales.<br />
Courtesy of<br />
Sandy<br />
Gardosik, PDA<br />
Figure 2. Chlorotic damage from scale feeding.<br />
Courtesy of Cathy Thomas, PDA<br />
Figure 3.<br />
Yellowed<br />
trees with<br />
Cryptomeria<br />
scale damage.<br />
Courtesy of<br />
Cathy Thomas,<br />
PDA<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
CRYPTOMERIA<br />
SCALE<br />
Aspidiotus cryptomeriae<br />
Kuwana<br />
Chlorotic damage from<br />
Cryptomeria scale feeding on<br />
the undersides of needles.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
CRYPTOMERIA SCALE ........................................................................................................................................................................................................................................................................... ..................................................................... 29 31
Figure 4. Adult male Cryptomeria<br />
scale emerged from its scale<br />
covering. Courtesy of Sandy<br />
Gardosik, PDA<br />
Figure 5. Adult female scale<br />
without its scale covering and<br />
other mature scales. Courtesy<br />
of Sandy Gardosik, PDA<br />
Figure 6. Exposed female scale<br />
with eggs. Courtesy of Sandy<br />
Gardosik, PDA<br />
After overwintering as immatures, the<br />
scales resume feeding and mature by late<br />
April. Males have an additional nonfeeding<br />
pupal stage be<strong>for</strong>e the winged male<br />
(Fig. 4) emerges and fl ies to a female (Fig.<br />
5). With the female under her protective<br />
cover, mating occurs and the male dies.<br />
Females deposit approximately 40 yellowish<br />
eggs beneath the cover be<strong>for</strong>e dying by late<br />
May or early June (Fig. 6). Crawlers emerge<br />
about 2 weeks after the eggs are deposited.<br />
The crawler emergence period may extend<br />
<strong>for</strong> 6–7 weeks but generally peaks about<br />
2–3 weeks after eggs are laid.<br />
Crawlers are relatively fragile and desiccate<br />
quickly (Fig. 7). They can crawl short<br />
distances but must settle and begin to feed<br />
within a day of emerging from the egg. As<br />
they settle and begin to feed, they create<br />
the waxy covering to protect themselves<br />
from environmental conditions (Fig. 8). It<br />
is not uncommon to fi nd a settled crawler<br />
beginning to develop its protective covering<br />
under the dead female. Several layers<br />
from different generations may develop with<br />
continual infestation.<br />
Figure 7.<br />
Crawler<br />
(circled),<br />
settled crawlers,<br />
female,<br />
and eggs.<br />
Courtesy of<br />
Sandy<br />
Gardosik, PDA<br />
Figure 8.<br />
Settled scale<br />
crawlers.<br />
Courtesy of<br />
Sandy<br />
Gardosik, PDA<br />
In summer, the stages repeat and by late<br />
July or early August, eggs of the second generation<br />
are present. The second-generation<br />
crawlers begin to emerge in mid-August,<br />
but some crawlers may be present into<br />
October. Two generations occur, but not all<br />
individuals mature at the same time, so the<br />
generations are not as distinct as some other<br />
multiple-generation pests.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant tree varieties that are less susceptible<br />
to Cryptomeria scale infestations.<br />
• Weed management in rows is important<br />
since it permits easier scouting and more<br />
thorough coverage if chemical controls<br />
are needed.<br />
Preseason<br />
• Scout <strong>for</strong> scale insects on the underside of<br />
needles, particularly those on the inside of<br />
bottom branches. Look <strong>for</strong> trees that exhibit<br />
yellow mottling on the upper surface<br />
of the needles (Fig. 9). If scale insects are<br />
found, verify that it is Cryptomeria scale<br />
by comparing the description with that<br />
of elongate hemlock scale. Male elongate<br />
hemlock scale coverings are white.<br />
• Scouting on an overcast day will allow<br />
symptoms to stand out.<br />
• Place sticky cards on branches showing<br />
symptoms to trap adult male scale insects<br />
(Fig. 10). Their emergence signals that<br />
eggs will soon follow.<br />
Figure 9. Lifting lower tree branches to scout<br />
<strong>for</strong> Cryptomeria scale. Courtesy of Cathy<br />
Thomas, PDA<br />
Figure 10. Sticky cards <strong>for</strong> monitoring adult<br />
male scale emergence. Courtesy of Cathy<br />
Thomas, PDA<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 32
• If only a few trees are infested, remove<br />
and destroy the trees be<strong>for</strong>e bud break.<br />
• If the block is generally infested, tag<br />
several trees that can be easily observed<br />
<strong>for</strong> crawler emergence.<br />
Growing Season<br />
• Growing degree days:<br />
— First-generation crawlers begin to<br />
emerge at 600–800 GDDs.<br />
— Second-generation crawlers begin to<br />
emerge at 1,750–2,130 GDDs.<br />
• Threshold level: Currently, no threshold<br />
level has been established. Cryptomeria<br />
scale can cause damage with just a few<br />
scales per needle.<br />
• Scout tagged trees <strong>for</strong> egg deposition in<br />
late May, and then scout daily <strong>for</strong> fi rst<br />
crawlers. Repeat scouting regime in late<br />
summer <strong>for</strong> second generation.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• Encourage naturally occurring parasitoids<br />
and predators.<br />
• Do not use a broad-spectrum insecticide<br />
that will kill benefi cial insects.<br />
Mechanical<br />
• Remove and destroy heavily infested trees<br />
be<strong>for</strong>e bud break. Wrap trees in a tarp/<br />
plastic when dragging them through the<br />
fi eld to prevent transferring scales to other<br />
trees.<br />
• Clean mower blades or tractors when<br />
moving them from an infected fi eld to an<br />
uninfected fi eld.<br />
• Butt-prune infested trees to remove the<br />
most heavily infested lower branches.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Apply dormant oil in spring be<strong>for</strong>e bud<br />
break. Thorough coverage of underside of<br />
lower branches is important.<br />
• For fi rst- or second-generation control,<br />
apply an appropriate spray targeting<br />
the crawlers after they are fi rst are seen.<br />
Repeat sprays every 7–10 days <strong>for</strong> control;<br />
up to four sprays may be required. Evaluate<br />
infested branches <strong>for</strong> presence of new<br />
crawlers after each spray to determine if<br />
another spray is necessary. Check needles<br />
from two to three seasons back <strong>for</strong> crawlers.<br />
Next Crop/Prevention<br />
• Purchase and plant scale-free nursery<br />
stock from a reputable company.<br />
• Remove heavily infested trees from a fi eld<br />
of new plantings as well as infected lower<br />
branches left behind after tree cutting.<br />
CRYPTOMERIA SCALE ............................................................................................................................................................................................................................................................................ 33
CYCLANEUSMA<br />
NEEDLE CAST<br />
Cyclaneusma minus<br />
(Butin) DiCosmo,<br />
Peredo, and Minter<br />
(syn. Naemacyclus<br />
minor Butin)<br />
Yellow-brown needles on<br />
infected tree. Courtesy of<br />
USDA Forest Service,<br />
Forest Health Protection, St.<br />
Paul Archive, Bugwood.org<br />
(#5050051)<br />
Hosts<br />
• Pines, esp. Scotch<br />
• Also reported on Austrian, Ponderosa,<br />
Mugo, and Monterey pines<br />
Damage Potential<br />
• Moderate<br />
Symptoms and Signs<br />
September Through November<br />
• Light green spots that eventually become<br />
yellow on needles 2 years old and older<br />
• Yellow needles with transverse brown<br />
bands<br />
October Through May<br />
• Symptomatic needles anywhere on the<br />
tree or cast to the ground<br />
• Off-white fruiting bodies that develop<br />
on yellow needles and swell with wet<br />
weather, making them easier to see<br />
Causes of Similar Symptoms<br />
• Aphid feeding<br />
• Other needle casts such as Lophodermium,<br />
Lophodermella, and Dothistroma<br />
• Environmental stresses<br />
• Air pollution<br />
• Fall needle drop<br />
• Winter injury<br />
• Pine needle scale<br />
Identifi cation<br />
Cyclaneusma needle cast is commonly referred<br />
to as the “fall yellower” of Scotch pines<br />
because of the timing and appearance of<br />
initial symptoms. Symptoms appear on older,<br />
interior needles from 10 to 15 months after<br />
infection occurs. In late summer or early fall,<br />
infected needles will have light green to yellow<br />
spots reminiscent of aphid or scale insect<br />
feeding. Gradually, these yellow spots enlarge<br />
to <strong>for</strong>m yellow bands, eventually causing the<br />
entire needle to turn yellow. By winter, the<br />
needles are light tan, and brown transverse<br />
bands become visible. Infected needles remain<br />
on the tree through the winter. Fruiting<br />
bodies <strong>for</strong>m about one month after symptoms<br />
appear. Because they are off-white in color<br />
and similar to the surrounding diseased tissue,<br />
they are diffi cult to detect. These structures<br />
become more obvious during periods of moist<br />
weather when they swell and split the epidermis<br />
of the needle, causing it to look like two<br />
open doors.<br />
Because many needle casts are similar,<br />
laboratory diagnosis may be warranted <strong>for</strong><br />
proper identifi cation be<strong>for</strong>e applying a<br />
fungicide.<br />
Biology and Life Cycle<br />
Cyclaneusma needle cast causes many pine<br />
species to prematurely defoliate. Some<br />
research suggests that this fungus may be an<br />
opportunistic pathogen that causes disease<br />
on plants that are under environmental<br />
stress. Four periods of infection occur <strong>for</strong><br />
this fungal disease. The fi rst is from mid-July<br />
through August, when the current year’s<br />
needles may become infected. September<br />
through November and late November<br />
through early December are the second and<br />
third infection periods. In Pennsylvania,<br />
these three periods account <strong>for</strong> about half of<br />
the new infections. The remaining 50 percent<br />
of infections occur from April through<br />
June, when both mature needles and new<br />
growth are susceptible.<br />
Spores are dispersed by the wind as they<br />
are released by fruiting bodies. If proper<br />
moisture is present, the fungus will enter<br />
the needle through stomata. About the<br />
time needles become discolored with brown<br />
bands, fruiting bodies will develop beneath<br />
the surface of the needle (Fig. 1). They begin<br />
to swell and the epidermis of the needle<br />
opens with a longitudinal fi ssure (Fig. 2).<br />
When moist or wet, the fruiting bodies<br />
enlarge and <strong>for</strong>cibly discharge spores.<br />
Figure 1. Yellow-brown bands on infected<br />
Scotch pine needles. Courtesy of Tracey Olson,<br />
PDA<br />
Figure 2. Swollen, light brown fruiting bodies<br />
that will rupture the surface of the needle.<br />
Courtesy of Joseph O’Brien, USDA Forest<br />
Service, Bugwood.org (#5050047)<br />
NEEDLE DISCOLORATION AND INJURY .......... ................................................................................................................................................................................................................................................ 34
Disease Cycle Calendar (Single Year’s Growth of Needles)<br />
May J J A S O N D Jan. F M A M J J A S O N D Jan. F M A M J J A S O N D<br />
Infection<br />
Symptoms<br />
Casting<br />
First Year Second Year Third Year<br />
Bud Break<br />
The heavier the shading, the more intense the infection/symptom/casting.<br />
Throughout the year, spores are released<br />
from infected needles that are attached or<br />
have fallen from the tree (Fig. 3) when the<br />
temperature is above freezing and there is<br />
moisture on the needle. Infected needles may<br />
remain on the tree or be cast anytime after<br />
they begin exhibiting symptoms (Fig. 4).<br />
Figure 3. Cast needles may continue to<br />
sporulate. Courtesy of Joseph O’Brien, USDA<br />
Forest Service, Bugwood.org (#5050046)<br />
Figure 4. Infected needles still attached to the<br />
tree. Courtesy of Joseph O’Brien, USDA Forest<br />
Service, Bugwood.org (#5050053)<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Choose a site that will promote drying<br />
of trees (southern slope, good drainage,<br />
nonshaded).<br />
• Provide adequate spacing between trees to<br />
allow <strong>for</strong> air circulation.<br />
• Plant stock that shows resistance or tolerance<br />
to the disease; use seed stock that<br />
exhibits genetic resistance.<br />
Preseason<br />
• Maintain proper nutrient and water levels<br />
to keep trees healthy and vigorous.<br />
• Scout trees in susceptible areas <strong>for</strong> any<br />
symptoms of the disease (a hand lens may<br />
be required).<br />
• Maintain proper weed control; do not<br />
allow weeds to grow up under or between<br />
the trees.<br />
Growing Season<br />
• Threshold level: If more than 20 percent<br />
of sampled trees have signs of the disease,<br />
consider treating the entire plantation.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
CYCLANEUSMA NEEDLE CAST .............................................................................................................................................................................................................................................................. 35
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Remove and destroy severely infected<br />
trees.<br />
• Remove dead needles beneath infected<br />
trees.<br />
• Examine needles around stumps <strong>for</strong> symptoms;<br />
remove infected needles if present.<br />
• Shear nondiseased trees prior to diseased<br />
trees to minimize spread of the fungus.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Fungicide application: In Pennsylvania,<br />
fi ve spray applications of an appropriate<br />
fungicide effectively treat the disease;<br />
spray in late March, early May, mid-June,<br />
mid-August, and early October.<br />
Next Crop/Prevention<br />
• Buy disease-free and/or resistant stock<br />
from a reputable nursery.<br />
• Scout in late fall <strong>for</strong> new trees just starting<br />
to show signs of infection.<br />
• Avoid planting next to old Scotch pine<br />
stands that may harbor disease.<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 36
Hosts<br />
• Douglas-fi r<br />
Damage Potential<br />
• Moderate–high<br />
Symptoms and Signs<br />
Summer Through Fall<br />
• Swollen areas on current year’s needles;<br />
needle may bend at gall<br />
• Pale or yellow spot(s) on both sides of<br />
needle; needle may turn purple or brown<br />
in fall<br />
• Premature needle loss<br />
Causes of Similar Symptoms<br />
• Rhabdocline needle cast<br />
• Cooley adelgid<br />
Identifi cation<br />
The Douglas-fi r needle midge is a tiny,<br />
orange-colored fl y, approximately 1 ⁄8 inch<br />
(5 mm) long. During the emergence period<br />
in spring, look <strong>for</strong> minute, orange fl ies resting<br />
on the tips of needles. Females can be<br />
distinguished by a very long ovipositor with<br />
which they probe between bud scales and<br />
into partially opened buds. This elongate<br />
ovipositor enables the female to deposit<br />
the long, narrow, orange-colored eggs in<br />
protected areas. A hand lens is needed to<br />
view the eggs.<br />
A white, legless maggot lacking a distinct<br />
head hatches from each egg and bores<br />
directly into the needle. In response to the<br />
maggot’s feeding, the needle produces excessive<br />
tissue, resulting in one or more swollen<br />
areas on the needle. There may be a minute,<br />
orange spot visible on the underside of the<br />
needle at the larval entrance site. When<br />
mature galls are carefully sliced open, a pale<br />
maggot may be found, generally under the<br />
entrance spot. At maturity, the maggot is<br />
about 1 ⁄8 inch (5 mm) long. After the maggot<br />
has dropped out of the needle to pupate<br />
Calendar of Activities<br />
in the ground, an irregular, three-sided exit<br />
hole can be seen in the epidermis on the<br />
underside of the affected needle. In fall and<br />
winter, damaged needles drop from the tree<br />
or break at the gall location, making winter<br />
scouting more diffi cult.<br />
Biology and Life Cycle<br />
Douglas-fi r needle midge overwinters as a<br />
mature larva in soil beneath an infested<br />
tree. In March–April, it pupates in the soil<br />
and the adult midge emerges (Fig. 1). The<br />
emergence period is generally from mid-<br />
April through early May and depends on the<br />
weather conditions at each site. Rain and<br />
cool temperatures delay emergence. Mating<br />
and oviposition occur immediately after<br />
emergence and adults die shortly thereafter<br />
(Fig. 2). The peak emergence period lasts<br />
about 7–10 days.<br />
Figure 1. Female<br />
Douglas-fi r needle<br />
midge. Courtesy<br />
of Sandy Gardosik,<br />
PDA<br />
Figure 2. Adult midge (circled) resting on a<br />
Douglas-fi r bud. Courtesy of Sandy Gardosik,<br />
PDA<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
*Place emergence traps during the monitoring time. Control measures occur based on trap catch.<br />
DOUGLAS-FIR<br />
NEEDLE MIDGE<br />
Contarinia pseudotsugae<br />
Condrashoff<br />
Kinking caused by<br />
Douglas-fi r needle midge<br />
larvae. Courtesy of Tracey<br />
Olson, PDA<br />
DOUGLAS-FIR NEEDLE MIDGE .............................................................................................................................................................................................................................................................. ..................................................................... 29 37
Figure 3. Eggs found tucked<br />
in the needle bud be<strong>for</strong>e buds<br />
are fully broken. Courtesy of<br />
Rayanne D. Lehman, PDA<br />
Figure 4. Young larva found<br />
inside a needle. Courtesy of<br />
Rayanne D. Lehman, PDA<br />
Figure 5. Early evidence of galls;<br />
needles are beginning to kink<br />
(late July). Courtesy of Tracey<br />
Olson, PDA<br />
Figure 6. Advanced gall<br />
symptoms (November).<br />
Courtesy of Tracey Olson, PDA<br />
Figure 7. Mature larva still in<br />
the needle (December).<br />
Courtesy of Tracey Olson, PDA<br />
Eggs (Fig. 3) hatch within a few days and<br />
larvae (Fig. 4) bore directly into the young<br />
needles to feed throughout the summer. As<br />
the larvae feed and grow, galls <strong>for</strong>m in the<br />
needles and cause them to kink (Fig. 5).<br />
By late summer, the galls will begin to<br />
turn yellow and then brown (Fig. 6). In late<br />
fall, the full-grown larvae (Fig. 7) exit the<br />
needles and drop to the ground to overwinter,<br />
leaving a distinct triangular exit hole<br />
in the underside of the needle (Fig. 8).<br />
A single generation occurs each year.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant late breaking tree varieties to<br />
decrease midge damage.<br />
• Remove overgrown Douglas-fi rs from the<br />
perimeter of the block. Damage is often<br />
more severe on older trees.<br />
Preseason<br />
• Place emergence traps under the north<br />
side of previously infested trees by April<br />
1 or be<strong>for</strong>e the daily temperature reaches<br />
60°F <strong>for</strong> several days (Fig. 9). See Appendix<br />
E: Insect Trap Use and Construction<br />
<strong>for</strong> in<strong>for</strong>mation on trap construction.<br />
• Place at least three traps per fi eld and<br />
check every day until the fi rst midge appears.<br />
Record the number of midges in the<br />
trap jar be<strong>for</strong>e emptying and replacing the<br />
jar. Continue monitoring until no midges<br />
are present <strong>for</strong> several days.<br />
Growing Season<br />
• Growing degree days: The adult midges<br />
emerge from the soil beneath trees at<br />
200–400 GDDs (based on midge population<br />
monitoring in Pennsylvania).<br />
• Threshold level: No established threshold<br />
level exists <strong>for</strong> this pest.<br />
• Scout in August <strong>for</strong> infestation. Mark<br />
trees that exhibit symptoms to aid with<br />
trap placement and distribution of control<br />
chemical next spring.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Figure 8. Needle<br />
showing larva<br />
exit hole.<br />
Courtesy of<br />
Tracey Olson,<br />
PDA<br />
Control Options<br />
Biological<br />
• Encourage and protect natural predators<br />
such as tiny parasitic chalcid wasps.<br />
Ill-timed chemical application is not<br />
effective and may kill natural enemies.<br />
Mechanical<br />
• Remove heavily infested trees in early fall<br />
be<strong>for</strong>e larvae exit the needles.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Base application on collection of adults<br />
in emergence trap. Chemicals will not be<br />
effective against eggs, only adults. First<br />
application should be made within a day<br />
of collection of the fi rst midge.<br />
• Consider a second application in 2 weeks<br />
if adults are continuing to emerge.<br />
Next Crop/Prevention<br />
• Purchase and plant pest-free nursery stock<br />
from a reputable company.<br />
Figure 9. Emergence trap placed under a<br />
previously infected tree. Courtesy of Sandy<br />
Gardosik, PDA<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 38
Hosts<br />
• Fir (Abies spp.)<br />
• Hemlock (Tsuga spp.)<br />
• Occasionally found on spruce (Picea spp.),<br />
Douglas-fi r (Pseudotsuga menziesii), and<br />
pines (Pinus spp.)<br />
Damage Potential<br />
• Low–high<br />
Symptoms and Signs<br />
• Yellowing of needles on the interior of the<br />
lower branches; damage moves upward as<br />
population increases<br />
• Scale coverings on the underside of the<br />
needles<br />
• Premature needle drop; eventual branch<br />
and limb dieback and death of tree with<br />
severe infestations<br />
• <strong>Tree</strong> may appear fl ocked<br />
Causes of Similar Symptoms<br />
• Spruce spider mite<br />
• Cryptomeria scale<br />
Identifi cation<br />
Elongate hemlock scale—sometimes<br />
referred to as Fiorinia scale—is an armored<br />
scale found more prevalently in southeastern<br />
Pennsylvania. Translucent, oval eggs<br />
occur beneath the scale covering of the<br />
female. The pale yellow crawlers are about<br />
1 ⁄250 inch (0.1 mm) long and have six short<br />
legs. When they settle to feed, the crawlers<br />
lose their legs and excrete an amber-colored,<br />
oval covering. Male and female scales develop<br />
differently and have different protective<br />
coverings. Immature males are about<br />
1 ⁄20 inch (1.0–1.5 mm) long and produce a<br />
whitened, waxy covering. Longer threads of<br />
wax are occasionally present and may give<br />
the covering a fuzzy appearance. Immature<br />
females are longer at 1 ⁄14 inch (1.5–2.0 mm)<br />
and produce a yellowish to orange-brown,<br />
parallel-sided covering. At maturity, the<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
delicate, light brown, male scales emerge<br />
and fl y to the sessile, wingless females.<br />
They may be mistaken <strong>for</strong> parasitoids as<br />
they crawl over the female coverings prior<br />
to mating.<br />
Biology and Life Cycle<br />
Elongate hemlock scale overwinters in<br />
several developmental stages in Pennsylvania<br />
and matures in spring (Figs. 1 and 2).<br />
In southern and Mid-Atlantic states, two<br />
overlapping generations have been reported,<br />
while in states in the Northeast, a single<br />
generation is known. In Pennsylvania,<br />
reproduction is staggered, so crawlers are<br />
present throughout the growing season.<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
Apply dormant oil prior to bud break.<br />
Figure 1. Heavy infestation of elongate hemlock<br />
scale. Courtesy of PDA<br />
Figure 2. Female (brown) and male (white)<br />
scales. Courtesy of Sandy Gardosik, PDA<br />
ELONGATE<br />
HEMLOCK<br />
SCALE<br />
Fiorinia externa Ferris<br />
Chlorotic damage from<br />
elongate hemlock scale<br />
feeding on the undersides of<br />
needles. Courtesy of Sandy<br />
Gardosik, PDA<br />
ELONGATE HEMLOCK SCALE ................................................................................................................................................................................................................................................................ ..................................................................... 29 39
Figure 3. Female scales and<br />
mobile yellow crawlers on the<br />
needles. Courtesy of Sandy<br />
Gardosik, PDA<br />
Figure 4. Chlorotic scale feeding<br />
damage on grand fi r. Courtesy<br />
of Cathy Thomas, PDA<br />
Figure 5. Female elongate<br />
hemlock scale. Courtesy of<br />
Sandy Gardosik, PDA<br />
Figure 6. Immature male<br />
elongate hemlock scales.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
The fi rst egg hatch generally starts in<br />
late May or early June. Mobile crawlers<br />
move from under the female’s covering to<br />
the underside of needles (Fig. 3) and begin<br />
to feed by inserting their piercing-sucking<br />
mouthparts. This feeding causes chlorotic,<br />
or yellow, spotting on the upper needle<br />
surface (Fig. 4). At the same time, the<br />
crawlers begin to secrete a translucent,<br />
waxy covering. Mature females are present<br />
about 6–8 weeks after emerging (Fig. 5).<br />
Male scales molt an additional time be<strong>for</strong>e<br />
the winged stage emerges (Figs. 6 and 7).<br />
Following mating, the males die and the<br />
female begins to produce about 12–16 eggs<br />
under her armored covering (Fig. 8) As a<br />
result of the various overwintering stages in<br />
Pennsylvania, crawlers emerge throughout<br />
the growing season, making repeated<br />
control ef<strong>for</strong>ts a necessity.<br />
Figure 7. Adult male elongate hemlock<br />
scale. Courtesy of Jim Stimmel, PDA<br />
Figure 8. Eggs inside the overturned female<br />
elongate hemlock scale casing. Courtesy of<br />
Sandy Gardosik, PDA<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant tree species that are not susceptible<br />
to elongate hemlock scale.<br />
• Properly space trees when planting to<br />
minimize infestation from tree to tree and<br />
enable thorough coverage if chemical<br />
controls are used.<br />
• Weed management is important to expose<br />
lower branches <strong>for</strong> ease of detection and<br />
effective control.<br />
• Remove and destroy any mature host trees<br />
that may serve as source of infestation.<br />
(Canada hemlock is a common host in<br />
Pennsylvania.)<br />
Preseason<br />
• Limit the use of nitrogen fertilizers; nitrogen<br />
enhances survival and developmental<br />
rate of scales.<br />
• Scout <strong>for</strong> elongate hemlock scale on the<br />
underside of needles. Note: Sometimes<br />
a combined population of Cryptomeria<br />
scale, balsam woolly adelgid, and hemlock<br />
scale can be found on the same trees.<br />
• If only a few infested trees are found,<br />
removing and destroying them be<strong>for</strong>e bud<br />
break may prevent a serious infestation<br />
from developing.<br />
• If numerous infested trees are located, tag<br />
a few to use <strong>for</strong> observations of crawler<br />
emergence.<br />
• Place sticky cards on branches showing<br />
symptoms to trap adult male scale insects<br />
(Fig. 9). Their emergence signals that eggs<br />
will soon follow.<br />
Figure 9. Yellow sticky cards used to attract<br />
the emerging male elongate hemlock scales.<br />
Courtesy of Cathy Thomas, PDA<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 40
Growing Season<br />
• Starting in mid-May, scout tagged trees<br />
every few days to check <strong>for</strong> crawler<br />
emergence.<br />
• Growing degree days: Crawlers are active<br />
from 360 to 700 GDDs.<br />
• Threshold level: No specifi c threshold<br />
level exists at this time. However, dieback<br />
of major limbs tends to occur by the time<br />
the population reaches ten scales per<br />
needle.<br />
• Continue scouting <strong>for</strong> crawlers every<br />
few weeks to determine the need <strong>for</strong><br />
additional controls.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• Encourage natural predators such as<br />
lady beetles, parasitic wasps, and several<br />
lacewing species.<br />
• Avoid applications of broad-spectrum<br />
insecticides that will kill natural<br />
predators.<br />
Mechanical<br />
• Remove and destroy heavily infested trees<br />
be<strong>for</strong>e bud break. Wrap trees in a tarp/<br />
plastic when dragging them through the<br />
fi eld to prevent transferring scales to other<br />
trees.<br />
• Clean mower blades or tractors when<br />
moving them from an infected fi eld to an<br />
uninfected fi eld.<br />
• Butt-prune infested trees to remove the<br />
most heavily infested lower branches.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Horticultural oil may be applied during<br />
the growing season. Use lower rate to<br />
avoid damage to new growth or wait to<br />
apply until new growth has hardened off.<br />
• When fi rst crawlers are active, apply a<br />
systemic insecticide spray three to four<br />
times over a 12-week period (three sprays<br />
four weeks apart or four sprays three weeks<br />
apart). Do not apply more than four times<br />
per season.<br />
• Evaluate each application to determine<br />
the need <strong>for</strong> subsequent sprays.<br />
Next Crop/Prevention<br />
• Purchase and plant scale-free nursery<br />
stock from a reputable company.<br />
ELONGATE HEMLOCK SCALE ................................................................................................................................................................................................................................................................. 41
ERIOPHYID<br />
RUST AND<br />
SHEATH MITES<br />
Nalepella and Setoptus<br />
spp.<br />
Lower branch showing rusty<br />
feeding damage of the<br />
spruce rust mite. Courtesy of<br />
Sandy Gardosik, PDA<br />
Hosts<br />
• Spruce<br />
• Fir<br />
• Eastern white pine<br />
• Scotch pine<br />
• Hemlock<br />
Damage Potential<br />
• Moderate<br />
Symptoms and Signs<br />
Rust Mites (Nalepella spp.)<br />
• Chlorotic or rusty-brown-appearing<br />
needles<br />
• Silvered, dusty, or bleached-out<br />
appearance on Colorado blue spruce<br />
• Premature needle drop may occur<br />
Sheath Mites (Setoptus spp.)<br />
• Stunted/chlorotic needles; premature<br />
needle drop<br />
• Silvering or graying of needles,<br />
particularly on south side of tree<br />
Causes of Similar Symptoms<br />
• Spruce spider mite<br />
• Environmental stress<br />
• Nutrient imbalances<br />
Identifi cation<br />
Eriophyid mites are distinctly different from<br />
the typical spider mite. They are extremely<br />
small and only have two pairs of legs in all<br />
active stages. The body is wedge or carrot<br />
shaped and light tan or pinkish (Figs. 1–3).<br />
Figure 1. Spruce rust mites<br />
in new growth. Courtesy of<br />
Sandy Gardosik, PDA<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Figure 2. Hemlock rust mites<br />
moving along the needle.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
Three species of rust mites in the genus<br />
Nalepella are found on <strong>Christmas</strong> trees in<br />
Pennsylvania. Hemlock rust mite, Nalepella<br />
tsugifolia Keifer, is the predominant species<br />
on fi r and hemlock. Another species, Nalepella<br />
octonema Keifer, is found occasionally<br />
on fi r. The third species, dubbed the spruce<br />
rust mite (Nalepella halourga Keifer), is<br />
restricted to spruce. Identifi cation of species<br />
requires examination under a very highpowered<br />
microscope. However, because the<br />
biology, life history, and damage of the three<br />
species are the same, confi rmation of a rust<br />
mite infestation is all that is needed.<br />
Two species of pine sheath mites in the<br />
genus Setoptus are found in Pennsylvania.<br />
White pine sheath mite, Setoptus strobacus<br />
Keifer, can be found in the needle sheath and<br />
on the needles of eastern white pine. Scotch<br />
pine sheath mite, Setoptus jonesi Keifer, has<br />
the same habits but on Scotch pine.<br />
To scout <strong>for</strong> eriophyid mites, use a<br />
15–20X hand lens. Select a lateral branch<br />
4–5 feet (1.23–1.52 m) from the ground on<br />
the southern-facing side of the tree. For<br />
rust mites, turn the branch over to see the<br />
underside of the needles and look <strong>for</strong> mites<br />
and eggs or cast skins. To locate sheath<br />
mites, look inside the needle sheath at the<br />
base of the needles. The mites are slow<br />
moving, and the anterior end is the widest<br />
portion of the body. You may see two pairs<br />
of short legs.<br />
Eggs are round and about the size of the<br />
needle stomata. During the growing season,<br />
eggs are generally found in the same location<br />
as the adults and are off-white or tan. Overwintering<br />
eggs will be found on the lower<br />
Figure 3. Wedge-shaped<br />
eriophyid mites (note four<br />
legs). Courtesy of Sandy<br />
Gardosik, PDA<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
NEEDLE DISCOLORATION AND INJURY .......... ................................................................................................................................................................................................................................................ 42
third of the needle (closer to the needle base<br />
on fi r) and, like the adults, on the underside<br />
of the needle (Fig. 4). These eggs range in<br />
color from clear to salmon to tan and can<br />
be deposited in clusters of 20 or more (Fig.<br />
5). To see these eggs, pull needles one by<br />
one and examine the base. Overwintering<br />
sheath mite eggs are located on the exposed<br />
base of needle bundles. During the growing<br />
season, these eggs will be found in the<br />
needle sheath and can be located by gently<br />
pulling needle bundles from the sheath.<br />
Figure 4. Overwintering<br />
rust<br />
mite eggs laid<br />
near the base<br />
of the needle.<br />
Courtesy of<br />
Sandy<br />
Gardosik, PDA<br />
Figure 5.<br />
Overwintering<br />
rust mite eggs<br />
laid in clusters<br />
of 20 or more.<br />
Courtesy of<br />
Rayanne D.<br />
Lehman, PDA<br />
Another method of detecting eriophyid<br />
mites is to look <strong>for</strong> cast skins on the needles.<br />
These appear as minute, white strands and<br />
offer better contrast to the needles than the<br />
adults. A hand lens is needed to look <strong>for</strong><br />
cast skins (Figs. 6 and 7). If this method is<br />
employed, an attempt to fi nd adults or eggs<br />
should follow to confi rm the infestation is<br />
active.<br />
Figure 6.<br />
Threadlike<br />
cast skins of<br />
eriophyid<br />
mites.<br />
Courtesy of<br />
Rayanne D.<br />
Lehman, PDA<br />
Figure 7.<br />
Cast skins<br />
giving the<br />
needles<br />
a fuzzy<br />
appearance.<br />
Courtesy of<br />
Sandy<br />
Gardosik,<br />
PDA<br />
Biology and Life Cycle<br />
All species of rust mites overwinter as eggs<br />
on the needles of host trees. Egg hatch<br />
occurs in very early spring, as early as mid-<br />
March. These are “cool-season” mites, like<br />
the spruce spider mite, but are active several<br />
weeks be<strong>for</strong>e spider mite eggs hatch. By the<br />
time spider mite overwintering eggs hatch,<br />
rust mites have completed several generations<br />
and rusty or bleached damage may be<br />
apparent (Fig. 8).<br />
During late spring and summer, rust mite<br />
populations are very low. However, in late<br />
summer when temperatures begin to moderate,<br />
populations build again. Overwintering<br />
eggs are deposited in fall, but rust mites may<br />
be active into December.<br />
White pine sheath mite biology may<br />
involve two distinctly different phases of<br />
activity. When candles are elongating, mites<br />
inhabit the needle sheath and feed on the<br />
elongating needles (Fig. 9). This causes<br />
needles to be stunted. The mites feed openly<br />
on mature needles, causing them to turn<br />
silver or brown.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• No recommendations are available<br />
at this time.<br />
Preseason<br />
• Begin scouting in early March by examining<br />
10–20 trees per acre. Choose trees<br />
with previous damage or, if no previous<br />
damage has occurred, trees at random.<br />
— On spruce, look <strong>for</strong> clusters of red eggs<br />
on underside of needle.<br />
— On fi r, look <strong>for</strong> amber-colored eggs<br />
along the base of the needle where it<br />
lies fl at against the stem.<br />
— On pine, look at the exposed needle<br />
base of last year’s growth.<br />
• Scouting on overcast days will help<br />
symptoms stand out.<br />
• Look <strong>for</strong> predatory mites that may be feeding<br />
on overwintering eggs or immature<br />
mites following egg hatch. Predatory mites<br />
may keep this pest in check.<br />
Figure 8. Mite feeding damage<br />
giving foliage a rusted or<br />
bleached look. Courtesy of<br />
Tracey Olson, PDA<br />
Figure 9. White pine sheath<br />
mites (circled). Courtesy of<br />
Rayanne D. Lehman, PDA<br />
ERIOPHYID RUST AND SHEATH MITES ................................................................................................................................................................................................................................................. 43
Growing Season<br />
• Growing degree days: Overwintering eggs<br />
hatch at 0–15 GDDs.<br />
• Threshold level (both criteria <strong>for</strong> rust<br />
mite on hemlock must be met):<br />
— At least 80 percent of sampled shoots<br />
have mites on them.<br />
— At least eight mites are present on<br />
one single needle in the plantation<br />
(examine all sides of the needle).<br />
• Continue scouting in spring until temperatures<br />
rise. Look <strong>for</strong> pine sheath mites by<br />
gently pulling elongating needles from the<br />
sheath and examining the needle bases.<br />
• Resume scouting in fall <strong>for</strong> population<br />
resurgence.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• Encourage predatory mites.<br />
Mechanical<br />
• No recommendations are available<br />
at this time.<br />
Biorational<br />
• Apply dormant oil in early spring be<strong>for</strong>e<br />
bud break. Eggs may have already hatched,<br />
but oil will control active mites if coverage<br />
is thorough. Oil will remove the blue color<br />
on some spruce.<br />
Chemical<br />
• Apply a miticide specifi cally labeled <strong>for</strong><br />
rust or eriophyid mites in mid-April–<br />
mid-May. General miticides may not<br />
control eriophyid mites. Make a second<br />
application if warranted 1–2 weeks later.<br />
• Scout to determine if a fall application is<br />
necessary.<br />
Next Crop/Prevention<br />
• Purchase and plant pest-free nursery stock<br />
from a reputable company.<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 44
Gypsy moths are of limited geographical<br />
distribution in the United States. They are<br />
responsible <strong>for</strong> signifi cant damage to many<br />
hardwood trees in the northeastern states.<br />
Regulations are in place <strong>for</strong> shipping cut<br />
trees from areas known to be infested with<br />
gypsy moth, including Pennsylvania. Contact<br />
the appropriate state or federal regulatory<br />
agency <strong>for</strong> in<strong>for</strong>mation be<strong>for</strong>e shipping<br />
cut trees out of state.<br />
Hosts<br />
• Many hardwood species<br />
• Some conifers: pines, spruce, eastern red<br />
cedar, and occasionally fi rs and Douglas-fi r<br />
Damage Potential<br />
• Low–moderate<br />
• Heaviest damage frequently caused by<br />
more mature larvae that have consumed<br />
all the food supply in the area; repeated<br />
light or a single severe defoliation of<br />
conifers usually results in tree mortality<br />
Symptoms and Signs<br />
• Defoliation of branches or entire tree<br />
(at fi rst noticeable at the top of the tree)<br />
• Tan egg masses on the trunk or underside<br />
of large branches<br />
• Gypsy moth caterpillars up to 2½ inches<br />
(63–64 mm) long<br />
• Reddish-brown pupal cases hanging from<br />
trunk<br />
• Adult moths in midsummer<br />
Causes of Similar Symptoms<br />
• None<br />
Identifi cation<br />
The oval, convex egg masses, up to 1½<br />
inches (40 mm) long, are a mixture of hundreds<br />
of roundish, tan eggs and buff-colored<br />
hairs from the abdomen of the female. They<br />
can be found year-round attached to any<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
hard surface such as tree trunks or branches,<br />
fence rows, siding of homes, or anything left<br />
outside <strong>for</strong> the summer. Healthy egg masses<br />
are lighter in color; emerged egg masses are<br />
darker and have numerous holes in the surface.<br />
Holes may be from emerging caterpillars<br />
or parasitoids.<br />
Newly emerged gypsy moth caterpillars<br />
are dark brown, ¼ inch (6 mm) long,<br />
and have longer hairs at each end, which<br />
results in the larvae appearing somewhat “I”<br />
shaped. As the larvae mature, the characteristic<br />
colored spots on the upper portion<br />
of the body become apparent. Each of the<br />
fi rst fi ve segments of the larva’s body has<br />
a pair of blue spots; the next six segments<br />
each have a pair of red spots. Between these<br />
spots, a thin, yellowish line runs the length<br />
of the body. The head of a mature caterpillar<br />
is black and tan mottled, and numerous<br />
long, brown hairs cover the body. Caterpillars<br />
reach 2½ inches in length (63–64 mm)<br />
at maturity.<br />
The dark reddish-brown to black pupal<br />
cases are not surrounded by any protective<br />
silk cocoon. They are attached to the substrate<br />
by several strands of silk and the cast<br />
skin from the last instar caterpillar may be<br />
nearby. Female pupal cases are larger than<br />
male pupal cases.<br />
The gray-brown male moths emerge fi rst.<br />
They have a wingspan of about 1½ inches<br />
(40 mm) and are agile fl iers. They can be<br />
recognized by their rapid, zigzag fl ight as<br />
they search <strong>for</strong> female moths. This makes<br />
close examination diffi cult, but the males<br />
have prominent feathered antennae, and<br />
their wings have irregular dark lines across<br />
the <strong>for</strong>ewings. The larger female moths<br />
have a wingspan of 2–2½ inches (50–63<br />
mm). Their wings are white with faint, dark<br />
markings. The heavy female’s body prevents<br />
fl ight, so they are usually found not far from<br />
their empty pupal cases.<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
GYPSY MOTH<br />
Lymantria dispar<br />
(Linnaeus)<br />
Severe gypsy moth damage<br />
on Colorado blue spruce.<br />
Courtesy of PDA<br />
GYPSY MOTH ........................................................................................................................................................................................................................................................................................ ..................................................................... 29 45
Figure 1. Gypsy moth egg mass.<br />
Courtesy of Pennsylvania DCNR<br />
Forestry Archive, Bugwood.org<br />
(#5020037)<br />
Figure 2. Larvae “ballooning,”<br />
or dropping down onto new<br />
hosts via silken thread.<br />
Courtesy of Brian Schildt, PDA<br />
Figure 3. Gypsy moth larvae<br />
and silk covering Douglas-fi r<br />
buds. Courtesy of Brian Schildt,<br />
PDA<br />
Figure 4. Maturing caterpillar<br />
with red and blue spots and<br />
yellowish lines running the<br />
length of the body. Courtesy of<br />
Brian Schildt, PDA<br />
Biology and Life Cycle<br />
Eggs overwinter in the egg masses protected<br />
somewhat by the hairs from the female’s<br />
abdomen (Figs. 1 and 6). The masses may<br />
contain up to 600 eggs, which are rounded<br />
and tan. Egg masses darken with age, and<br />
hatching occurs between late April and<br />
mid-May when oak leaves are expanding.<br />
Newly hatched larvae (caterpillars) remain<br />
on the egg mass <strong>for</strong> a few days be<strong>for</strong>e moving<br />
to the newly developing leaves. If the<br />
population is high or the host is not suitable<br />
<strong>for</strong> development, larvae will “balloon” to<br />
new hosts by dropping down from a silken<br />
thread (Figs. 2 and 3). Spring winds can<br />
carry the young caterpillars up to a mile.<br />
During this phase, gypsy moth larvae are<br />
frequently seen on clothing when working<br />
outdoors. The irritating hairs of the fi rst<br />
instar caterpillars may cause contact<br />
dermatitis <strong>for</strong> some people.<br />
Larvae feed <strong>for</strong> 6–7 weeks, gradually<br />
increasing in size and appetite. Young larvae<br />
remain in the tree’s foliage day and night,<br />
but their feeding pattern changes when they<br />
are half grown (Fig. 4). The more mature<br />
larvae move down the tree to take refuge in<br />
a cool, shady place during the day and feed<br />
in the canopy at night.<br />
By late June to early July, the larvae fi nd<br />
a sheltered place to pupate (Fig. 5). About<br />
2 weeks are required be<strong>for</strong>e the adults begin<br />
to emerge, with the male moths appearing<br />
several days be<strong>for</strong>e the females. When<br />
the female moths emerge, they may crawl<br />
a short distance to an elevated spot or stay<br />
near the pupal case to emit a pheromone<br />
to attract the male moths. After mating occurs,<br />
females deposit one to two egg masses<br />
be<strong>for</strong>e dying (Fig. 6). Neither male nor<br />
female moths feed during their short lives.<br />
Egg masses overwinter until the following<br />
spring. One generation is produced per year<br />
in Pennsylvania.<br />
Figure 5. Gypsy moth pupae suspended from<br />
tree limbs. Courtesy of PDA<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant the crop away from hardwood stands<br />
such as aspen or oak or other preferred<br />
hosts of the gypsy moth.<br />
Preseason<br />
• Scout year-round <strong>for</strong> gypsy moth egg<br />
masses both in the plantation and in<br />
surrounding woodlots.<br />
• Monitor egg masses <strong>for</strong> fi rst signs of<br />
hatching.<br />
• Contact a regulatory agency or county<br />
extension offi ce to obtain in<strong>for</strong>mation<br />
about specifi c management options in the<br />
area and about shipping <strong>Christmas</strong> trees.<br />
Growing Season<br />
• Growing degree days: 90–448 GDDs is<br />
the treatment window <strong>for</strong> the larvae.<br />
• When larvae are small, consider<br />
spraying with a microbial or other type<br />
of insecticide.<br />
• To help monitor populations, use a<br />
synthesized female pheromone trap to<br />
look <strong>for</strong> male moth emergence.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Figure 6. Female<br />
gypsy moth over<br />
egg mass. Courtesy<br />
of Hannes Lemme,<br />
Bugwood.org<br />
(#1260007)<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 46
Control Options<br />
Biological<br />
• Encourage natural predators of gypsy<br />
moths in all life cycle stages: egg parasitoid<br />
(Ooencyrtus kuvanae Howard),<br />
parasitoid of larva (Blepharipa pratensis<br />
Meigen and Meteorus pulchricornis<br />
Wesmael), predatory beetle (Calosoma<br />
sycophanta Linneaus), birds, mice, shrews,<br />
and ground beetles.<br />
• “Wilt” is a naturally occurring virus that<br />
is always present in the larvae but doesn’t<br />
kill them until they become stressed due<br />
to overcrowding or reduced food supply.<br />
• The fungal insect pathogen Entomophaga<br />
maimaiga can help decrease heavy populations<br />
during wet spring weather.<br />
Mechanical<br />
• In fall, winter, or spring, be<strong>for</strong>e eggs<br />
hatch, scrape egg masses off the trees<br />
or other areas where they are located.<br />
Destroy the eggs by burning or soaking<br />
them in soapy water <strong>for</strong> several days.<br />
Biorational<br />
• Use a microbial insecticide such as Bt<br />
(Bacillus thuringiensis) when larvae are less<br />
than 1 inch (25 mm) long <strong>for</strong> maximum<br />
control.<br />
Chemical<br />
• Several insecticides are registered <strong>for</strong><br />
this pest. Read and follow all labels<br />
accordingly.<br />
Next Crop/Prevention<br />
• Purchase and plant pest-free nursery stock<br />
from a reputable company.<br />
GYPSY MOTH ......................................................................................................................................................................................................................................................................................... 47
LOPHODERMIUM<br />
NEEDLE CAST<br />
Lophodermium<br />
seditiosum Minter,<br />
Staley, and Millar<br />
Lophodermium needle<br />
cast, the “spring reddener,”<br />
infection on a fi eld of pine.<br />
Courtesy of Edward L.<br />
Barnard, Florida Department<br />
of Agriculture and Consumer<br />
Services, Bugwood.org<br />
(#4823057)<br />
Hosts<br />
• Scotch, Austrian, and red pines most<br />
susceptible<br />
• Most two- and three-needled pines<br />
Damage Potential<br />
• Moderate on fi eld-grown trees<br />
• High on seedlings<br />
Symptoms and Signs<br />
• May fi rst develop in needles on lower<br />
branches<br />
Late Winter Through Early Spring<br />
• Infected needles develop yellow or<br />
reddish-brown spots<br />
• Current season’s infected needles redden<br />
by early spring<br />
Late Spring Through Midsummer<br />
• Needles brown and may fall from tree,<br />
leaving only new, healthy-appearing<br />
growth<br />
• Raised, black, football-shaped fruiting<br />
bodies appear on dead needles still<br />
attached to the tree and those on the<br />
ground<br />
Causes of Similar Symptoms<br />
• Winter burn<br />
• Dothistroma needle blight<br />
• Lethale needle cast<br />
• Cyclaneusma needle cast<br />
• Pine needle scale<br />
Identifi cation<br />
The most accurate and easiest identifi cation<br />
period <strong>for</strong> Lophodermium needle cast<br />
is in mid- to late summer when the fruiting<br />
bodies are present on needles infected the<br />
previous year. The shiny, black, footballshaped<br />
fruiting bodies are 1 ⁄32 inch (0.8 mm)<br />
long. They are slightly raised and aligned<br />
lengthwise on the needle. When mature,<br />
they have a longitudinal slit through which<br />
spores will be released. To see if fruiting<br />
bodies are mature, wrap some symptomatic<br />
needles in a moist paper towel <strong>for</strong> about<br />
20 minutes to see if they open and release<br />
spores. Fruiting bodies occur on dead<br />
needles remaining on the tree or those<br />
already on the ground.<br />
This common fungal disease is often referred<br />
to as the “spring reddener” of Scotch<br />
pines. In spring, needles infected the previous<br />
year will have small, brown spots, often<br />
with yellow margins. The most noticeable<br />
symptoms occur as the season progresses<br />
and needles turn yellow and eventually<br />
reddish brown. They may be cast anytime<br />
after symptoms appear. Fruiting bodies are<br />
required to confi rm a diagnosis.<br />
Biology and Life Cycle<br />
Needle infection starts in mid- to late summer<br />
and only the current year’s growth is<br />
susceptible. Spores released from mature<br />
fruiting bodies (Fig. 1) are dispersed by<br />
wind or water to the new growth or to other<br />
trees. The fungus enters the healthy needles<br />
through stomata and begins to disrupt the<br />
moisture-distribution mechanism in the<br />
needles. Symptoms are generally not apparent<br />
until early spring of the year following<br />
infection. At this time, infected needles begin<br />
to change color, fi rst yellowing and then<br />
turning reddish brown by the time shoots are<br />
elongating (Fig. 2). Severe infections will<br />
Figure 1. Lophodermium fruiting<br />
bodies. Courtesy of Tracey Olson, PDA<br />
Figure 2. Infected needles from the previous<br />
year turn reddish brown and drop in the spring.<br />
Courtesy of USDA Forest Service Northeastern<br />
Archive, Bugwood.org (#1398006)<br />
NEEDLE DISCOLORATION AND INJURY .......... ................................................................................................................................................................................................................................................ 48
cause an entire tree to appear scorched, with<br />
only small, green shoots appearing at the<br />
end of branches. As the disease progresses,<br />
infected needles generally drop from the<br />
tree, leaving only the healthy, new growth.<br />
By late summer, fruiting bodies are mature<br />
and can be found on cast needles under<br />
the tree, lodged in branches, and on the few<br />
browned needles remaining on the branches<br />
(Fig. 3). When moisture is present, the<br />
fruiting bodies split longitudinally to release<br />
minute spores. This often occurs during cool<br />
rain events in late summer and fall. Since<br />
most of the infection comes from needles<br />
already on the ground, poor air circulation<br />
under the tree is a major contributor to the<br />
spread of the disease. The infection from<br />
needles already cast from the tree is unique<br />
to Lophodermium. Most other fungal diseases<br />
are spread from structures remaining on the<br />
tree.<br />
Figure 3. Characteristic football-shaped<br />
Lophodermium fruiting body. Courtesy<br />
of Tracey Olson, PDA<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Choose a site that will promote drying<br />
of trees (southern slope, good drainage,<br />
nonshaded).<br />
• Do not plant near windbreaks of other<br />
susceptible conifers.<br />
• Remove infected trees around the block;<br />
rake and remove disease-bearing needles<br />
under infected trees.<br />
• Adequately space trees when planting to<br />
encourage drying and minimize disease<br />
transmission.<br />
• Avoid planting short-needled “Spanish”<br />
Scotch pine and “French Green” varieties<br />
since they are very susceptible to infection.<br />
• Examine all seedlings be<strong>for</strong>e planting<br />
and cull any suspected of being infected.<br />
Buy from a reputable producer.<br />
Preseason<br />
• Control weeds around and under trees to<br />
allow <strong>for</strong> good air circulation and drying<br />
of foliage.<br />
• In very early spring, scout <strong>for</strong> yellowing<br />
of needles. Needles often have small,<br />
brown spots with yellow margins that<br />
increase with time. Tag any suspect trees<br />
to examine later <strong>for</strong> reddening of needles.<br />
• Examine dead needles under suspect<br />
trees <strong>for</strong> evidence of fruiting bodies from<br />
previous year.<br />
Growing Season<br />
• Scout <strong>for</strong> symptoms of Lophodermium in<br />
May and June by examining the lower<br />
branches of at least 50 trees of varying<br />
ages scattered throughout the block. Tag<br />
several suspect trees to watch <strong>for</strong> fruiting<br />
body production.<br />
• Threshold level: If at least 10 percent of<br />
the examined trees show injury, consider<br />
treating the entire plantation.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Disease Cycle Calendar (Single Year’s Growth of Needles)<br />
May J J A S O N D Jan. F M A M J J A S O N D Jan. F M A M J J A S O N D<br />
Infection<br />
Symptoms<br />
Casting<br />
First Year Second Year Third Year<br />
Bud Break<br />
The heavier the shading, the more intense the infection/symptom/casting.<br />
LOPHODERMIUM NEEDLE CAST ............................................................................................................................................................................................................................................................ 49
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• In spring, remove and destroy severely<br />
infected trees. Rake and remove any<br />
fallen needles and branches.<br />
• After harvest, examine needles around<br />
fresh stumps. If fruiting bodies are found,<br />
remove and destroy debris.<br />
• Avoid tip-ups (live branches on stumps)<br />
that may contain infected needles.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Apply an appropriate fungicide three to<br />
four times beginning in July and continuing<br />
at three-week intervals. If early<br />
summer is warm and/or wet weather is<br />
prolonged, adjust the timing and number<br />
of the sprays accordingly.<br />
• Apply the fungicide during periods of low<br />
air movement and when material will<br />
have adequate time to dry on needles.<br />
Next Crop/Prevention<br />
• Inspect plants/nursery stock and buy<br />
from a reputable company.<br />
• Collect seeds from trees showing<br />
resistance <strong>for</strong> a seed-collection program.<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 50
Hosts<br />
• Pines, especially Scotch and Mugo<br />
• Occasionally on Douglas-fi r, true fi rs,<br />
and spruce<br />
Damage Potential<br />
• High on Scotch pine<br />
• Low on other hosts<br />
Symptoms and Signs<br />
Throughout the Year<br />
• White “fl ecks” on needles; white,<br />
oyster-shell-shaped scales that are<br />
generally heaviest on lower branches<br />
• Yellowing or brown needles leading to<br />
premature needle drop<br />
• Heavy infestations give a frosted or silvery<br />
appearance<br />
• Untreated infestations may result in death<br />
of twigs, branches, and eventually the tree<br />
Causes of Similar Symptoms<br />
• Needle cast diseases<br />
Identifi cation<br />
Pine needle scale is an armored scale that<br />
produces a white, oyster-shell-shaped, wax<br />
covering. This characteristic covering is<br />
easiest to fi nd on overcast days. The covering<br />
of the mature scale measures 1 ⁄16– 1 ⁄8 inch<br />
(2.5–3.0 mm) in length and has a small,<br />
yellow spot on the narrow end. Male scale<br />
insects are fragile, gnatlike, and capable<br />
of short fl ight but are rarely seen. Female<br />
pine needle scale insects are tan with<br />
legless, wingless, fl attened bodies beneath<br />
the white covering. Males do not feed.<br />
Females and immature scales have hairlike<br />
piercing mouthparts used to suck sap from<br />
needles. Pine needle scales do not produce<br />
honeydew as they feed.<br />
The red, oval eggs are found beneath<br />
the covering of the female. Newly hatched<br />
nymphs, or crawlers, are paprika colored.<br />
Crawlers must molt and settle to feed a<br />
short time after emerging from the egg.<br />
After they settle, the mouthpart is inserted<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
into the needle, preventing further movement.<br />
Settled nymphs are yellowish to tan.<br />
After a very short period, they begin to<br />
produce the characteristic white covering.<br />
Biology and Life Cycle<br />
Pine needle scale has two generations per<br />
year in Pennsylvania. Be<strong>for</strong>e dying in fall,<br />
each female deposits approximately 40 eggs<br />
(Fig. 1). These oval, red eggs overwinter<br />
under the dead female’s covering and begin<br />
hatch by mid- to late May. Egg hatch and<br />
subsequent crawler emergence may occur<br />
over a period of 2–3 weeks. The fl attened,<br />
red crawlers have legs but lack mouthparts<br />
(Fig. 2). They move around on the needle<br />
and must settle and molt in a short time.<br />
Crawlers may fall from needles or be blown<br />
by air currents to infest neighboring trees.<br />
They can also be carried by insects, animals<br />
(including people), and machinery.<br />
Figure 1. Overwintering pine needle<br />
scale eggs with dead female scale.<br />
Courtesy of Sandy Gardosik, PDA<br />
Figure 2. Pine needle scale crawlers.<br />
Courtesy of Sandy Gardosik, PDA<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
PINE NEEDLE<br />
SCALE<br />
Chionaspis pinifoliae<br />
(Fitch)<br />
White fl ecks on Scotch pine<br />
needles (signs of a pine<br />
needle scale infestation).<br />
Courtesy of Cathy Thomas,<br />
PDA<br />
PINE NEEDLE SCALE .............................................................................................................................................................................................................................................................................. ..................................................................... 29 51
Figure 3. Adult female pine<br />
needle scale. Courtesy of PDA<br />
Figure 4. Mature pine needle<br />
scale population. Courtesy of<br />
Cathy Thomas, PDA<br />
Figure 5. Twice-stabbed lady<br />
beetle feeding on pine needle<br />
scales. Courtesy of Rayanne D.<br />
Lehman, PDA<br />
Settled crawlers insert their long mouthparts<br />
into the needle and begin to secrete<br />
the armored covering. Initially, the covering<br />
is clear or yellow, but eventually the characteristic<br />
white covering is produced (Fig. 3).<br />
This generation feeds on the previous year’s<br />
growth and can readily be hidden by new<br />
growth in spring.<br />
By July, both winged males and wingless<br />
females are present. Following mating, egg<br />
laying, and hatching, the crawlers resulting<br />
from this generation move onto the current<br />
year’s needles and settle to feed. This generation<br />
is most noticeable (Fig. 4). By September,<br />
the second generation is complete and<br />
females begin to deposit overwintering eggs.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Properly space trees; allow enough space<br />
between saleable trees.<br />
• Remove and destroy any mature trees<br />
in and around the plantation acting as a<br />
source <strong>for</strong> infestation.<br />
Preseason<br />
• Scout <strong>for</strong> scales.<br />
— On an overcast day, look <strong>for</strong> scale<br />
coverings on lower branches. Pry off<br />
the scale covering with a pin and use<br />
a hand lens to look <strong>for</strong> overwintering<br />
eggs. If found, mark the tree to monitor<br />
<strong>for</strong> egg hatch.<br />
— Ragged chew holes or round holes in<br />
the scale covering indicate parasitoid<br />
or predator activity.<br />
• An application of dormant horticultural<br />
oil on infested trees may provide little to<br />
moderate success controlling overwintering<br />
eggs. Note: Oil will remove the blue<br />
color from blue spruces.<br />
Growing Season<br />
• Growing degree days:<br />
— First-generation crawlers appear at<br />
298–448 GDDs.<br />
— Second-generation crawlers appear<br />
at 1,290–1,917 GDDs.<br />
• Threshold level: No specifi c threshold<br />
in<strong>for</strong>mation is available at this time.<br />
Populations on Scotch and Mugo pines<br />
are capable of rapid increase, but those on<br />
other hosts are generally not signifi cant.<br />
• Treat if tree growth is stunted, yellowing<br />
occurs, or if scale populations become<br />
unsightly or aesthetically displeasing.<br />
Spot treat infested trees instead of treating<br />
the entire plantation.<br />
• Methods of scouting <strong>for</strong> crawlers (early<br />
May and early July):<br />
— Visit trees marked during late winter or<br />
early spring to look <strong>for</strong> crawler emergence.<br />
— Wrap electrical tape, sticky side out,<br />
around twigs with high populations<br />
of scales; monitor daily <strong>for</strong> the fi rst<br />
crawler to emerge.<br />
— Hold a white piece of paper under a<br />
branch while shaking the branch.<br />
Look <strong>for</strong> crawlers on the paper.<br />
• While scouting <strong>for</strong> crawlers, always scout<br />
<strong>for</strong> predators (several lady beetle species<br />
such as twice-stabbed lady beetle,<br />
lacewings, etc.) and wasp parasitoids or<br />
evidence of their presence.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• Encourage natural predators and<br />
parasitoids to assist with managing small<br />
infestations (Fig. 5).<br />
• If possible, reduce insecticide use <strong>for</strong><br />
other pests.<br />
• Larger infestations may not be controlled<br />
with predators alone.<br />
Mechanical<br />
• Prune and destroy heavily infested<br />
branches; prune branches that are in<br />
danger of coming into contact with<br />
one another.<br />
• Do not mow or remove infested trees<br />
during crawler emergence as this may<br />
spread crawlers.<br />
Biorational<br />
• Insect growth regulators (IGRs) can be<br />
used during the crawler stage to disrupt<br />
the molting process.<br />
Chemical<br />
• Apply a spray targeting crawlers <strong>for</strong> 2–3<br />
weeks at 7-day intervals after the fi rst<br />
crawler is seen (most effective after egg<br />
hatch and be<strong>for</strong>e development of white<br />
wax covering). Note: This poses a risk<br />
to benefi cial predators and parasitoids.<br />
Whenever possible, use a “softer,<br />
predator-friendly” insecticide.<br />
• Systemic insecticides can be effective<br />
against young, settled nymphs that have<br />
just begun feeding (June and August).<br />
Next Crop/Prevention<br />
• Purchase and plant scale-free nursery<br />
stock from a reputable company.<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 52
Hosts<br />
• European and redheaded pine sawfl ies:<br />
Scotch, red, Mugo, Jack, and Austrian<br />
pines<br />
• Introduced pine sawfl y: eastern white pine<br />
Damage Potential<br />
• Moderate<br />
Symptoms and Signs<br />
• Complete defoliation or sparse, patchy,<br />
missing foliage anywhere on the tree<br />
• Needles may appear brown, wilted, and<br />
strawlike or twisted, as if singed<br />
• Clusters of young larvae feeding on<br />
needles<br />
Causes of Similar Symptoms<br />
• None<br />
Identifi cation<br />
Adult pine sawfl ies are seldom seen. They<br />
are related to and resemble bees in size and<br />
shape. They have two pairs of transparent<br />
wings but are not capable of stinging.<br />
Instead of a stinger, the female has a sawlike<br />
ovipositor that she uses to make a slit in the<br />
edge of a needle. She deposits a single egg<br />
into each slit and several eggs in a needle.<br />
The larvae are caterpillar-like with six<br />
or more pairs of prolegs on the abdomen.<br />
Moth and butterfl y caterpillars have fi ve or<br />
fewer prolegs. Both types of larvae also have<br />
three pairs of jointed true legs. Larvae use<br />
their chewing mouthparts to consume entire<br />
needles, which can result in extensive<br />
defoliation.<br />
Young larvae feed in colonies or clusters<br />
and can defoliate entire trees, depending on<br />
the size of the colony and tree. A distinguishing<br />
habit of the larvae is that they<br />
collectively rear back when a hand is waved<br />
over the cluster.<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
Scout <strong>for</strong> European pine sawfl y eggs in needles.<br />
European Pine Sawfl y<br />
Larvae are dull gray green with a shiny<br />
black head (Fig. 1). They have light green<br />
and black stripes running the length of the<br />
body and are capable of growing to approximately<br />
1 inch (25 mm) long. Larvae feed on<br />
previous year’s growth, causing a decrease<br />
in growth rate but not tree death since they<br />
do not attack current growth. Young larvae<br />
eat only the outside of old growth, leaving<br />
the needles brown and strawlike behind<br />
the green, current growth. Older larvae eat<br />
the entire needle, leaving behind only the<br />
needle sheath. The adults are brown, fl ylike<br />
insects that are ½ inch (12 mm) long.<br />
Introduced Pine Sawfl y<br />
Larvae are black or dark brown with two<br />
dark stripes down the back and yellow and<br />
white patches on the sides. The head is<br />
black and shiny, and the underside is pale<br />
yellow or white (Fig. 2). They can grow up<br />
Figure 1. Colony of feeding European pine<br />
sawfl y larvae. Courtesy of Steven Katovich,<br />
USDA Forest Service, Bugwood.org (#5369986)<br />
Figure 2. Introduced pine sawfl y larva.<br />
Courtesy of Sandy Gardosik, PDA<br />
PINE SAWFLIES<br />
European Pine Sawfl y,<br />
Neodiprion sertifer<br />
(Geoffroy)<br />
Introduced Pine Sawfl y,<br />
Diprion similis (Hartig)<br />
Redheaded Pine<br />
Sawfl y, Neodiprion<br />
lecontei (Fitch)<br />
Feeding damage from<br />
European pine sawfl y.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
PINE SAWFLIES ...................................................................................................................................................................................................................................................................................... ..................................................................... 29 53
Figure 3. Colony of redheaded<br />
pine sawfl y larvae. Courtesy of<br />
Sandy Gardosik, PDA<br />
Figure 4. European pine sawfl y<br />
eggs. Courtesy of Rayanne D.<br />
Lehman, PDA<br />
Figure 5. Colony of feeding<br />
European pine sawfl y larvae.<br />
Courtesy of Rayanne D. Lehman,<br />
PDA<br />
Figure 6. European pine sawfl y<br />
adult. Courtesy of Louis-Michel<br />
Nageleisen, Département de la<br />
Santé des Forêts, Bugwood.org<br />
(#2102003)<br />
to 1 inch (25 mm) long. The larvae feed in<br />
groups when young and singly as they mature.<br />
Depending on the generation, larvae<br />
may eat old needles or new growth. The<br />
cocoon of the introduced pine sawfl y is a<br />
strong, brown, semiglossy, textured cylinder.<br />
Adults are chunky and have black heads<br />
and thoraxes. Males are ¼ inch (7 mm)<br />
long with brown or black abdomens, and<br />
females are 1 ⁄3 inch (8 mm) long with black<br />
and yellow abdomens.<br />
Redheaded Pine Sawfl y<br />
Newly hatched larvae are white, unspotted,<br />
and have a brown or black head. As larvae<br />
feed and mature, they develop a red head<br />
and two to four rows of brown/black spots<br />
on the yellow body (Fig. 3). The last segment<br />
has a larger black patch on its sides.<br />
They can grow to 1 inch (25 mm) long.<br />
This species prefers younger trees in shaded<br />
areas. The larvae eat old needles fi rst, but<br />
heavy infestation or additional generations<br />
will cause new growth to be eaten as<br />
well. Young larvae eat the outside of the<br />
needles, leaving behind brown, strawlike<br />
needles, while older larvae consume the<br />
entire needle. The cocoon is a papery, yet<br />
tough, brown cylinder with rounded ends.<br />
Adult females are reddish brown and have a<br />
black abdomen with white spots. Males are<br />
slender and black with feathery antennae.<br />
Females tend to be larger than males.<br />
Biology and Life Cycle<br />
The three common pine sawfl ies in Pennsylvania<br />
have similar life cycles that differ<br />
in the overwintering stage, timing of egg<br />
hatch, and number of generations each year.<br />
Each species deposits eggs inside slits created<br />
by females in needles. Only fertilized<br />
eggs will result in females; unfertilized eggs<br />
produce males. When larvae are mature,<br />
they produce capsulelike cocoons in which<br />
they pupate.<br />
European Pine Sawfl y<br />
European pine sawfl ies overwinter as yellow<br />
eggs deposited in the needles (Fig. 4). The<br />
eggs hatch in April through mid-May. Larvae<br />
feed as a colony (10–100 larvae) and eat<br />
previous year’s growth through July (Fig. 5).<br />
Three to four larvae may be seen feeding on<br />
a single needle. When the larvae are fully<br />
grown, they drop to the ground and pupate<br />
around mid-August to early September.<br />
Adults emerge in mid- to late September<br />
and mate (Fig. 6). They only live a few<br />
days and do not feed. Using the sawlike<br />
ovipositor, females lay 6–8 eggs per needle<br />
and may use 10–12 needles <strong>for</strong> oviposition.<br />
One generation occurs per year.<br />
Introduced Pine Sawfl y<br />
Introduced pine sawfl ies overwinter as<br />
prepupae in cocoons on the ground in leaf<br />
litter. They pupate in early spring, with<br />
adults emerging in May through early June.<br />
Some prepupae may exhibit diapause, meaning<br />
they may overwinter additional seasons<br />
be<strong>for</strong>e becoming adults. Adults mate, and<br />
the females lay light bluish-colored eggs in<br />
the needles and cover them with a green,<br />
frothy substance. Females each lay approximately<br />
70 eggs with 10 eggs per needle<br />
(Fig. 7). Eggs hatch in roughly 2 weeks and<br />
colonies of larvae begin eating old-growth<br />
needles. As the larvae mature, they feed<br />
solitarily until fully mature (Fig. 8). Around<br />
early July, larvae spin cocoons and pupate a<br />
Figure 7. Introduced pine sawfl y eggs.<br />
Courtesy of John H. Ghent, USDA Forest<br />
Service, Bugwood.org (#0488043)<br />
Figure 8. Mature introduced pine sawfl y larva<br />
feeding solitarily. Courtesy of PDA<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 54
short time afterward. Cocoons can be found<br />
among the needles, at the base of branches,<br />
and within bark crevices (Fig. 9). Adults<br />
emerge, peaking in early August. They mate<br />
and again lay eggs. These eggs hatch around<br />
one week later, and the larvae begin feeding<br />
on old- and current-growth needles. In<br />
September, the larvae drop to the ground<br />
to overwinter as prepupae. Two generations<br />
normally occur per year, although a partial<br />
or full third generation can occur if weather<br />
conditions are favorable.<br />
Redheaded Pine Sawfl y<br />
Similar to the introduced pine sawfl ies, the<br />
redheaded pine sawfl ies overwinter as prepupae<br />
and may exhibit diapause. They pupate<br />
in spring and adults emerge a few weeks<br />
later. Females deposit approximately 100<br />
eggs, which hatch around one month later.<br />
Larvae feed in colonies <strong>for</strong> 5–6 weeks. They<br />
begin feeding on old growth fi rst but will<br />
also consume new growth during a heavy<br />
infestation (Fig. 10). When mature, the<br />
larvae drop to the ground and spin a cocoon<br />
to overwinter. In some southern locations, a<br />
second generation is produced in the same<br />
season. In those cases, fi rst-generation larvae<br />
feed <strong>for</strong> a shorter time be<strong>for</strong>e pupating and<br />
creating the second generation. Populations<br />
of redheaded pine sawfl ies tend to be somewhat<br />
cyclic. Outbreaks of high populations<br />
resulting in heavy defoliation <strong>for</strong> several<br />
years are followed by periods of low populations<br />
with little damage.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant nonpine species that are not<br />
susceptible to sawfl y damage.<br />
Preseason<br />
• In winter and early spring, inspect trees<br />
<strong>for</strong> European pine sawfl y eggs deposited<br />
in the needles. Tag trees to monitor <strong>for</strong><br />
larvae.<br />
• Remove trees that are larger than marketable<br />
size so they don’t serve as reservoirs<br />
<strong>for</strong> sawfl ies.<br />
Growing Season<br />
• Encourage natural predators.<br />
• Scout <strong>for</strong> young larvae feeding on needles<br />
beginning in May. Look <strong>for</strong> strawlike,<br />
brown needles and missing foliage throughout<br />
the season.<br />
• Growing degree days: European pine<br />
sawfl ies emerge at 78–220 GDDs.<br />
• Threshold level:<br />
— For introduced pine sawfl ies and<br />
redheaded pine sawfl ies, treatment may<br />
be warranted if more than 50 random<br />
trees in a young plantation have 10<br />
larvae per tree or if they exhibit sawfl y<br />
damage. Treat older plantations when<br />
damage level will hurt sale.<br />
— For European pine sawfl ies, treatment<br />
may be warranted if there is a large infestation<br />
and the tree is within 3 years<br />
of harvest.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• Birds, rodents, parasites, viruses, and other<br />
predators can help decrease sawfl y populations<br />
but are often not enough to manage<br />
the pest in a plantation setting.<br />
Mechanical<br />
• Prune infested branches of trees not ready<br />
<strong>for</strong> harvest if plantation infestation is<br />
small.<br />
• Remove larvae by hand and squash or<br />
place them in soapy water <strong>for</strong> several days<br />
(small infestations only).<br />
Biorational<br />
• Apply a horticultural oil or insecticidal<br />
soap labeled <strong>for</strong> control of sawfl ies when<br />
larvae are very young.<br />
Chemical<br />
• Spot treat young larval infestations with<br />
a registered virus or insecticide labeled<br />
<strong>for</strong> sawfl y control. Note: Bt (Bacillus<br />
thuringiensis) will not control sawfl ies.<br />
• Apply an insecticide labeled <strong>for</strong> sawfl y<br />
control to entire plantation when young<br />
larvae are present if more than 25 percent<br />
of trees are infested.<br />
• Chemical treatment may be needed more<br />
than once per season, depending on the<br />
species of sawfl ies.<br />
Next Crop/Prevention<br />
• Purchase and plant pest-free nursery stock<br />
from a reputable company.<br />
Figure 9. Introduced pine<br />
sawfl y cocoon on twig.<br />
Courtesy of Rayanne D.<br />
Lehman, PDA<br />
Figure 10. Feeding damage<br />
from redheaded pine sawfl y<br />
larvae. Courtesy of Sandy<br />
Gardosik, PDA<br />
PINE SAWFLIES ....................................................................................................................................................................................................................................................................................... 55
PLOIODERMA<br />
NEEDLE CAST<br />
Ploioderma lethale<br />
(Dearn.) Darker<br />
Symptoms of needles<br />
infected with Ploioderma<br />
needle cast. Courtesy of<br />
Tracey Olson, PDA<br />
Hosts<br />
• Austrian and red pines<br />
• Other two- or three-needled, hard-pine<br />
species<br />
Damage Potential<br />
• Low–moderate<br />
Symptoms and Signs<br />
• May fi rst develop in needles on lower<br />
branches<br />
Late Winter Through Early Spring<br />
• Infected needles develop reddish-brown<br />
spots<br />
• Spots may girdle needle and kill tips,<br />
leaving healthy, green base<br />
• Current season’s infected needles redden<br />
by early spring<br />
Late Spring Through Early Summer<br />
• In June, fruiting bodies may develop<br />
within spots and dead portion of needle;<br />
fruiting bodies appear as long ( 2 ⁄ 125– 1 ⁄ 5<br />
inch; 0.4–5.0 mm), black lines<br />
• Dead tips of infected needles with green<br />
bases may break off<br />
• Completely infected needles are cast<br />
Causes of Similar Symptoms<br />
• Other needle cast diseases, especially<br />
Lophodermium needle cast<br />
Identifi cation<br />
Ploioderma needle cast is a disease of hard<br />
pines. Although the infection occurs in<br />
spring, symptoms are not apparent until<br />
winter when yellow spots and bands appear,<br />
giving the needles a mottled appearance.<br />
During the following spring, the spots and<br />
bands girdle the needle, turning the tip<br />
straw brown or gray in color while the base<br />
of the needle remains green. Black, longitudinal<br />
fruiting bodies appear in the infected<br />
portion of the needles in June. Sporulation<br />
occurs after they mature, and the needles<br />
are cast to the ground by the end of summer.<br />
Ploioderma needle cast closely resembles<br />
Lophodermium needle cast in symptoms<br />
and disease cycle. However, Lophodermium<br />
fruiting bodies are elliptical to round, while<br />
those of Ploioderma are elongate, running<br />
longitudinally on the needle.<br />
Biology and Life Cycle<br />
During wet spring and early summer weather,<br />
colorless spores are released from fruiting<br />
bodies that have opened lengthwise on the<br />
needle (Fig. 1). Spores can be disseminated<br />
by wind or rain and will germinate on newly<br />
emerging needles close by. The fungus<br />
enters the needle through the stomata and<br />
the fi rst symptoms of infection are visible on<br />
the new growth by winter (Fig. 2). The next<br />
spring, fruiting bodies release spores and the<br />
process begins again.<br />
Figure 1. Infected needles with characteristic<br />
elongate, black fruiting bodies. Courtesy of<br />
Tracey Olson, PDA<br />
Figure 2. Early infection of Ploioderma needle<br />
cast. Courtesy of Joseph O’Brien, USDA Forest<br />
Service, Bugwood.org (#5057098)<br />
NEEDLE DISCOLORATION AND INJURY .......... ................................................................................................................................................................................................................................................ 56
Disease Cycle Calendar (Single Year’s Growth of Needles)<br />
May J J A S O N D Jan. F M A M J J A S O N D Jan. F M A M J J A S O N D<br />
Infection<br />
Symptoms<br />
Casting<br />
First Year Second Year Third Year<br />
Bud Break<br />
The heavier the shading, the more intense the infection/symptom/casting.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Choose a site that will promote drying<br />
of trees (southern slope, good drainage,<br />
nonshaded).<br />
• Plant resistant varieties suited <strong>for</strong> the<br />
environmental conditions.<br />
• Inspect nursery stock be<strong>for</strong>e buying and<br />
planting.<br />
• Adequately space trees to ensure air<br />
circulation and reduce moisture levels.<br />
Preseason<br />
• Maintain good weed control.<br />
• Scout needles in early spring <strong>for</strong> signs and<br />
symptoms of Ploioderma.<br />
Growing Season<br />
• If Ploioderma is found in the plantation,<br />
consider treating with a fungicide in the<br />
spring during bud break.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Promptly remove and destroy branches<br />
and trees showing signs of Ploioderma.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• To protect new growth in the spring,<br />
apply a fungicide when new growth<br />
is 2 inches long and needles are just<br />
beginning to emerge from the fascicles.<br />
• Two additional fungicide applications may<br />
be necessary at 14- to 21-day intervals to<br />
protect needles during the spore release<br />
and infection period.<br />
Next Crop/Prevention<br />
• Purchase and plant disease-free nursery<br />
stock from a reputable company.<br />
PLOIODERMA NEEDLE CAST .................................................................................................................................................................................................................................................................. 57
RED-BAND<br />
(DOTHISTROMA)<br />
NEEDLE BLIGHT<br />
Mycosphaerella pini<br />
Rostr.<br />
Anamorph:<br />
Dothistroma septospora<br />
(Doroguine) Morelet<br />
Girdling lesions banding the<br />
needle. Courtesy of Tracey<br />
Olson, PDA<br />
Hosts<br />
• Austrian, Ponderosa, and Mugo pines<br />
highly susceptible<br />
• Scotch and red pines generally resistant<br />
Damage Potential<br />
• Moderate<br />
Symptoms and Signs<br />
Late Summer Through Early Fall<br />
• Dark green bands on recently infected<br />
needles; bands may contain yellow or<br />
tan spots<br />
• Brown or reddish-brown bands and lesions<br />
on needles several weeks after infection<br />
Late Fall Through Winter<br />
• Brown, dead needle tips with the base of<br />
the needle remaining green<br />
• Dark brown or black, tiny fruiting bodies<br />
in the dead portion of the needle<br />
Late Winter Through Early Spring<br />
• Previously green base turns brown<br />
• Premature needle drop occurs (most<br />
severe on lower branches)<br />
Causes of Similar Symptoms<br />
• Brown spot needle blight<br />
• Lophodermium and Cyclaneusma needle<br />
casts<br />
• Pine needle scale<br />
• Winter injury<br />
• Environmental stress<br />
Identifi cation<br />
This disease, also referred to as Dothistroma<br />
needle blight, is called red-band needle<br />
blight because of the most common symptom<br />
found in the fi eld. Although initial<br />
symptoms include dark green bands on the<br />
needles, these are quickly replaced with<br />
brown or reddish-brown lesions. Only the<br />
base of the needle will be green, with the<br />
remaining portion tan or brown. Eventually,<br />
tiny, dark brown or black fruiting bodies are<br />
produced and release spores. The fruiting<br />
bodies are visible in the diseased area with a<br />
hand lens and are typically covered by a fl ap<br />
of needle epidermis.<br />
Biology and Life Cycle<br />
Red-band needle blight infection occurs<br />
throughout the growing season during<br />
wet periods. Beginning in May, spores are<br />
released from dark brown or black fruiting<br />
bodies that rupture through the epidermis<br />
of previously infected needles (Fig. 1). The<br />
spores are spread by wind and splashing<br />
rain and enter healthy needles through<br />
the stomata. Second-year or older needles<br />
are susceptible to infection throughout<br />
the season. Current-year needles are not<br />
susceptible until they have elongated and<br />
hardened off, usually around July. Infection<br />
from cast needles on the ground below the<br />
tree is negligible.<br />
Figure 1. Fruiting body rupturing the<br />
epidermis. Courtesy of Tracey Olson, PDA<br />
After infection takes place, symptoms<br />
are not visible <strong>for</strong> 3–6 months on average.<br />
In late summer, infected needles may show<br />
dark green bands that look water soaked.<br />
This symptom occurs briefl y and is usually<br />
not detected. Next, yellow to tan spots become<br />
visible and then eventually change to<br />
become larger, reddish-brown lesions banding<br />
the needle (Fig. 2). The fungus produces<br />
a toxin that quickly kills the tissue at the<br />
infection site and causes it to turn purplish<br />
red, giving this disease its common name,<br />
red-band needle blight. Girdling lesions<br />
will result in needle tips dying while the<br />
bases of the needles remain green (Fig. 3).<br />
By late fall, tiny, black fruiting bodies may<br />
appear in the bands or dead tissue. These<br />
will not mature and release spores until the<br />
following spring.<br />
NEEDLE DISCOLORATION AND INJURY .......... ................................................................................................................................................................................................................................................ 58
Disease Cycle Calendar (Single Year’s Growth of Needles)<br />
May J J A S O N D Jan. F M A M J J A S O N D Jan. F M A M J J A S O N D<br />
Infection<br />
Symptoms<br />
Casting<br />
First Year Second Year Third Year<br />
Bud Break<br />
The heavier the shading, the more intense the infection/symptom/casting.<br />
Figure 2. Reddish-brown lesions banding<br />
the needle. Courtesy of Tracey Olson, PDA<br />
Figure 3. Dead needle tips resulting from<br />
girdling by lesions. Courtesy of Tracey<br />
Olson, PDA<br />
Needle casting begins in fall and continues<br />
through late summer of the following<br />
year. The fi rst to drop are the second-year<br />
and older needles that were infected in<br />
spring (Fig. 4). Needles infected in the same<br />
year they are produced are usually not cast<br />
until late summer of the following year.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant resistant or tolerant varieties such as<br />
Scotch or red pine.<br />
• Plant in an area with good drainage and<br />
airfl ow.<br />
• Plant trees with adequate spacing to allow<br />
<strong>for</strong> airfl ow.<br />
Preseason<br />
• Control weeds in and around trees.<br />
• Remove and destroy lower whorls of<br />
branches to increase air circulation.<br />
• Scout <strong>for</strong> yellow needle spots, dead needle<br />
tips, and fruiting bodies in late fall or early<br />
spring; use a 15X hand lens to see the<br />
fruiting bodies.<br />
Growing Season<br />
• If symptoms of disease are present, consider<br />
treating the plantation. Severely<br />
infected trees provide a good source <strong>for</strong><br />
inoculum and should be removed.<br />
• Do not shear trees in wet weather.<br />
Shearing trees when foliage is wet may<br />
further spread the disease.<br />
• If disease is present in a block, shear<br />
disease-free stock fi rst.<br />
• Maintain weed control practices.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• No recommendations are available<br />
at this time.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Apply an appropriate protective fungicide<br />
once in mid-May to protect older foliage<br />
and again in mid-July to protect needles<br />
of all ages.<br />
Next Crop/Prevention<br />
• Purchase and plant disease-free nursery<br />
stock from a reputable company.<br />
Figure 4. Infected previous<br />
years’ needles will soon be<br />
cast. Courtesy of USDA Forest<br />
Service Archive, Bugwood.org<br />
(#2251050)<br />
RED-BAND (DOTHISTROMA) NEEDLE BLIGHT ...................................................................................................................................................................................................................................... 59
RHABDOCLINE<br />
NEEDLE CAST<br />
Rhabdocline weirii<br />
A. K. Parker and J. Reid<br />
Rhabdocline<br />
pseudotsugae Syd.<br />
Foliage with Rhabdocline<br />
needle cast infection.<br />
Courtesy of Tracey Olson,<br />
Host<br />
• Douglas-fi r<br />
Damage Potential<br />
• Moderate–high<br />
Symptoms and Signs<br />
• May fi rst develop in needles on lower<br />
branches<br />
Late Summer<br />
• Yellow spots or fl ecks on current-year<br />
needles that enlarge with time<br />
Late Winter Through Early Spring<br />
(Be<strong>for</strong>e Bud Break)<br />
• Reddish-brown spots on upper surface<br />
of current-year needles; distinct border<br />
between diseased area and healthy, green<br />
tissue<br />
• Swollen, light tan fruiting bodies on the<br />
underside of symptomatic needles<br />
Bud Break<br />
• Fruiting bodies rupture underside of<br />
needle epidermis, releasing mass of<br />
orange spores<br />
Late Spring (After Bud Break)<br />
• Darkened fruiting bodies indicating<br />
sporulation has passed<br />
Summer Through Early Fall<br />
• Previous year’s infected needles drop or<br />
cast; severely diseased trees might only<br />
retain current-year needles<br />
Causes of Similar Symptoms<br />
• Cooley spruce gall adelgid<br />
• Swiss needle cast<br />
• Douglas-fi r needle midge<br />
Identifi cation<br />
Douglas-fi r is the only known host of<br />
Rhabdocline. Laboratory analysis provides<br />
the most accurate identifi cation, but growers<br />
can generally identify this fungus in their<br />
fi elds by observing symptoms prior to bud<br />
break. In late winter or very early spring,<br />
look <strong>for</strong> reddish-brown splotches on the<br />
upper needle surface (Fig. 1). There is a<br />
distinct division between diseased tissue<br />
and the surrounding healthy, green tissue.<br />
As bud break approaches, remove shoots<br />
containing suspect needles and place them<br />
in a glass of warm water <strong>for</strong> a few minutes.<br />
Mature fruiting bodies of Rhabdocline will<br />
rupture the epidermis longitudinally on the<br />
underside of the needles and release a mass<br />
of orange spores.<br />
Biology and Life Cycle<br />
Rhabdocline needle cast infection occurs<br />
around bud break, when buds are opening to<br />
expose susceptible immature needles (Fig. 2).<br />
During periods of high humidity (rain shower<br />
or heavy morning dew), the mature fruiting<br />
bodies open (Fig. 3). Fruiting bodies are only<br />
found on the previous year’s needles and<br />
open on the underside of the needle. During<br />
spore release, the needle epidermis splits<br />
lengthwise, revealing a light tan to orange<br />
spore mass. Individual spores are dispersed<br />
by water and wind to the susceptible new<br />
growth. Spores germinate on the wet needle<br />
and penetrate the cuticle be<strong>for</strong>e the fungus<br />
begins to grow inside the needle.<br />
PDA Figure 1. Distinct symptomatic<br />
reddish-brown splotches of<br />
Rhabdocline needle cast (March).<br />
Courtesy of Tracey Olson, PDA<br />
Figure 2. Early bud break of<br />
Douglas-fi r buds signaling the<br />
start of Rhabdocline sporulation.<br />
Courtesy of Brian Schildt, PDA<br />
Figure 3. Fruiting bodies rupture<br />
on the undersides of Rhabdoclineinfected<br />
needles during bud break.<br />
Courtesy of Tracey Olson, PDA<br />
NEEDLE DISCOLORATION AND INJURY .......... ................................................................................................................................................................................................................................................ 60
Disease Cycle Calendar (Single Year’s Growth of Needles)<br />
May J J A S O N D Jan. F M A M J J A S O N D Jan. F M A M J J A S O N D<br />
Infection<br />
Symptoms<br />
Casting<br />
First Year Second Year Third Year<br />
Bud Break<br />
The heavier the shading, the more intense the infection/symptom/casting.<br />
The infection period may last several<br />
weeks, but only the newly emerging spring<br />
growth can become infected. When the<br />
fruiting body turns dark brown or black,<br />
spore production is complete <strong>for</strong> the year<br />
(Fig. 4.) Needles containing spent fruiting<br />
bodies will be cast from the tree through<br />
late spring and summer. <strong>Tree</strong>s that have<br />
severe infection over consecutive years may<br />
hold only the most current year’s needles<br />
during the winter (Fig. 5).<br />
Figure 4. Brown to black fruiting<br />
bodies signaling that spore production<br />
is complete <strong>for</strong> the year.<br />
Courtesy of Tracey Olson, PDA<br />
Figure 5. Severely infected tree with only<br />
current-year needles remaining. Courtesy of<br />
Brian Schildt, PDA<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Choose a site with slope, good air circulation,<br />
and water drainage to promote<br />
drying of trees.<br />
• Properly space trees to encourage<br />
adequate drying of needles, especially<br />
in spring.<br />
• Plant resistant or tolerant tree varieties;<br />
avoid Douglas-fi rs from Rocky Mountain<br />
seed sources.<br />
• Remove and destroy unmanaged Douglasfi<br />
r that may act as a source of inoculum.<br />
Preseason<br />
• Maintain proper weed control year-round.<br />
• Concentrate scouting on trees planted in<br />
areas that are more conducive <strong>for</strong> disease<br />
development (low-lying or shaded areas).<br />
Look at all sides of the tree.<br />
• Scout the block in late winter or early<br />
spring (be<strong>for</strong>e bud break) <strong>for</strong> reddishbrown<br />
blotches on upper needle surface of<br />
most current-year needles. Overcast days<br />
af<strong>for</strong>d the best opportunity to see needle<br />
discoloration. Pay particular attention to<br />
needles on the lower third of the tree and<br />
trees that look thin and only have last<br />
year’s needles. If symptomatic trees are<br />
located, tag several to observe <strong>for</strong> maturation<br />
of fruiting bodies.<br />
• Soak suspect needles in warm water to<br />
check <strong>for</strong> the maturity level of the fruiting<br />
bodies. When fruiting bodies open, spores<br />
are mature and ready to be released.<br />
Growing Season<br />
• Threshold level: Ask a state/regional plant<br />
inspector about any regulatory thresholds.<br />
• In late April/early May, begin regular<br />
scouting <strong>for</strong> bud break in each target<br />
block. Make the fi rst fungicide application<br />
when the fi rst buds begin to open.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
RHABDOCLINE NEEDLE CAST ................................................................................................................................................................................................................................................................ 61
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Remove and destroy severely infected<br />
trees prior to bud break.<br />
• Prune and remove dead/dying branches<br />
when they are dry.<br />
• Butt-prune large trees to encourage drying<br />
of lower branches.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Apply an appropriate fungicide when buds<br />
begin to break on the trees in the block.<br />
Do not wait <strong>for</strong> new growth to elongate.<br />
Successful prevention of infection depends<br />
on preventing spores from infecting new<br />
needles—keep a protective coating of<br />
fungicide on the new growth <strong>for</strong> this to<br />
happen.<br />
• Follow through with two to three additional<br />
applications until needles are fully<br />
elongated and mature or fruiting bodies<br />
have darkened. See the schedule below:<br />
— First application: when fi rst trees in<br />
plantation break bud<br />
— Second application: 1 week after fi rst<br />
application<br />
— Third application: 2 weeks after second<br />
application<br />
— Fourth application: 3 weeks after third<br />
application only if spring is prolonged<br />
by cool, wet weather and sporulation<br />
remains active or Swiss needle cast is<br />
detected<br />
Next Crop/Prevention<br />
• Purchase and plant disease-free nursery<br />
stock from a reputable company.<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 62
Hosts<br />
• Colorado blue spruce and Engelmann<br />
spruce most susceptible<br />
• White spruce occasionally susceptible<br />
Damage Potential<br />
• Moderate<br />
Symptoms and Signs<br />
• May fi rst develop in needles on lower<br />
branches<br />
• Older needles may exhibit discoloration<br />
throughout the year<br />
Late Fall Through Early Spring<br />
• Yellow mottling of fi rst-year needles<br />
Early Spring (Be<strong>for</strong>e Bud Break)<br />
• Yellow needles turn brown or purplebrown;<br />
black fruiting bodies with white<br />
cap protruding from needle stomata<br />
develop<br />
Late Spring (After Bud Break)<br />
• Initial casting of previous year’s infected<br />
needles<br />
Summer Through Early Fall<br />
• Casting of previous year’s infected<br />
needles, leading to large bare areas on<br />
trees; severely diseased trees might only<br />
retain current-year needles<br />
Causes of Similar Symptoms<br />
• Spruce spider mite<br />
• Cytospora canker<br />
• Flyspeck<br />
• Environmental stress (drought)<br />
• Nutritional defi ciencies<br />
• Spray damage<br />
Identifi cation<br />
Rhizosphaera needle cast disease is caused<br />
by the fungus Rhizosphaera kalkhoffi i Bubak.<br />
Colorado and Engelmann spruce are very<br />
susceptible, but Norway and white spruce<br />
are somewhat resistant. Young trees sustain<br />
the most severe damage, but trees of any<br />
size can be affected. Field identifi cation is<br />
based on symptoms, but accurate identifi cation<br />
requires laboratory analysis.<br />
Healthy spruce trees will retain 5 or<br />
more years of needles. Those infected with<br />
Rhizosphaera may have only one year of<br />
needles. Foliage on inner branches and on<br />
the lower portion of the tree is more prone<br />
to infection since these areas tend to remain<br />
wet <strong>for</strong> longer periods. When the infection<br />
is severe, this disease will kill lower branches<br />
and infection will progress to the upper<br />
branches.<br />
Yellowing needles of any age or any that<br />
are reddish brown to purple in fall are suspect.<br />
Look particularly at trees that appear<br />
thin on the lower half or are only retaining<br />
1–2 years of needle growth. Examine apparently<br />
healthy needles on these trees in early<br />
spring, prior to bud break. Look <strong>for</strong> minute,<br />
black fruiting bodies, 1 ⁄250 inch (0.1 mm) in<br />
diameter, in neat lines running the length of<br />
the needle.<br />
Biology and Life Cycle<br />
Rhizosphaera overwinters in infected needles<br />
on the tree. In spring, the fruiting bodies<br />
mature and push through the stomata. They<br />
appear as neat lines of tiny, black spots<br />
about the size of the stomata (Fig. 1). About<br />
the time of bud break, they mature, and<br />
when there is adequate moisture, spores are<br />
released. The spores are splashed by rain to<br />
the new growth, where infection will begin.<br />
The spores enter the stomata of the healthy<br />
needle and germinate after a minimum of<br />
48 hours. Under less favorable conditions,<br />
longer germination time is required. Most<br />
infection occurs in spring, but fi rst-year<br />
needles are susceptible throughout the<br />
growing season. In some areas of Pennsylvania,<br />
a second infection period in August and<br />
September has been reported.<br />
The disease continues to develop in<br />
the needle <strong>for</strong> the next year, but symptoms<br />
are generally not well expressed. This long<br />
period between infection and appearance<br />
of symptoms makes this disease diffi cult to<br />
diagnose and control. Around May, one<br />
year following infection, apparently healthy<br />
Figure 1. Fruiting bodies pushing up through<br />
the stomata. Courtesy of Tracey Olson, PDA<br />
RHIZOSPHAERA<br />
NEEDLE CAST<br />
Rhizosphaera kalkhoffi i<br />
Bubak<br />
Rhizosphaera-infected<br />
foliage on inner branches<br />
and lower parts of the tree.<br />
Courtesy of USDA Forest<br />
Service North Central<br />
Research Station Archive,<br />
Bugwood.org (#1406191)<br />
RHIZOSPHAERA NEEDLE CAST ............................................................................................................................................................................................................................................................. ..................................................................... 29 63
Disease Cycle Calendar (Single Year’s Growth of Needles)<br />
May J J A S O N D Jan. F M A M J J A S O N D Jan. F M A M J J A S O N D<br />
Infection<br />
Symptoms<br />
Casting<br />
First Year Second Year Third Year<br />
Bud Break<br />
The heavier the shading, the more intense the infection/symptom/casting.<br />
Figure 2. Apparently healthy<br />
needles bearing fruiting bodies.<br />
Courtesy of Tracey Olson, PDA<br />
Figure 3. Infected reddishbrown<br />
needle in fall. Courtesy<br />
of Tracey Olson, PDA<br />
Figure 4. Striking symptomatic<br />
color on Colorado blue spruce.<br />
Courtesy of USDA Forest<br />
Service Archive, Bugwood.org<br />
(#2634062)<br />
needles will bear mature fruiting bodies<br />
(Fig. 2). During summer, these now previous<br />
year’s needles will turn yellow and gradually<br />
reddish brown or purple by fall (Fig. 3).<br />
This discoloration is very striking, particularly<br />
on Colorado spruce with bright blue<br />
color (Fig. 4). Most infected needles are cast<br />
in the fall, but some infected needles will<br />
remain on the tree.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant on a slope with good drainage.<br />
• Plant resistant varieties such as Norway<br />
or white spruce. Plant disease-free stock.<br />
• Adequately space trees when planting<br />
to allow <strong>for</strong> air circulation and drying<br />
of needles.<br />
Preseason<br />
• Maintain proper weed control throughout<br />
season by mowing grass and brush.<br />
• Scout <strong>for</strong> fruiting bodies in spring be<strong>for</strong>e<br />
bud break.<br />
— Randomly select at least 20 trees to<br />
sample. Concentrate scouting on<br />
trees planted in areas that are more<br />
conducive <strong>for</strong> disease development.<br />
— Using a 10X hand lens, examine the<br />
2-year-old needles found on three<br />
lower branches of each tree sampled.<br />
Look <strong>for</strong> black fruiting bodies<br />
protruding from stomata openings.<br />
— During dry weather, remove and<br />
destroy infected branches and trees.<br />
Growing Season<br />
• If at least half of the sampled branches<br />
have fruiting bodies on 10 percent of<br />
needles, consider treating the entire<br />
plantation with a fungicide application.<br />
• Shear healthy trees fi rst during dry<br />
conditions.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Cut infected branches back to the main<br />
trunk.<br />
• Do not leave live branches on the stumps<br />
of harvested trees.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Apply an appropriate fungicide when<br />
new shoots are ¾–1¼ inches long (needles<br />
are half elongated) and a second spray<br />
3 weeks later (needles are fully elongated).<br />
Moderately infected trees may require<br />
2 years of fungicide applications.<br />
• In some areas, it may be necessary to make<br />
another application in mid-August to<br />
early September to prevent late summer<br />
infection.<br />
Next Crop/Prevention<br />
• Maintaining tree health and vigor can<br />
help guard against severe Rhizosphaera<br />
needle cast infection.<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 64
Hosts<br />
• Most spruce species susceptible<br />
• In Pennsylvania, most commonly reported<br />
on Colorado blue spruce and its varieties,<br />
Serbian spruce, and Sitka spruce<br />
Damage Potential<br />
• Moderate–high<br />
Symptoms and Signs<br />
Midsummer<br />
• Pale yellow bands become visible on<br />
current-year needles<br />
• Needles infected in previous year turn<br />
brown and defoliate<br />
Late Summer<br />
• Pale yellow bands with orange fruiting<br />
bodies (called telia) obvious on most<br />
recent growth; band will go entirely<br />
around needle and increase in color<br />
intensity as season progresses; discolored<br />
bands easiest to see on overcast days<br />
Bud Break<br />
• Bright orange telia mature and rupture on<br />
infected needles<br />
Throughout the Year<br />
• Infected trees may appear disfi gured and<br />
have extensive needle discoloration,<br />
reduced growth, and premature needle<br />
drop<br />
Causes of Similar Symptoms<br />
• Mechanical damage from needle<br />
punctured by needle tips<br />
• Cinara aphids<br />
• <strong>Pest</strong>icide phototoxicity<br />
Identifi cation<br />
Weir’s cushion rust (Chrysomyxa weirii) is the<br />
only species of spruce needle rust currently<br />
found in Pennsylvania. Infected current-year<br />
needles will exhibit pale yellow bands that<br />
go completely around the needle. Orange,<br />
immature fruiting structures may be visible<br />
within the band. This is easiest to see on<br />
an overcast day or on the shady side of the<br />
tree. Mechanical damage from pricking of<br />
needles during windy weather may mimic<br />
spruce needle rust. But, mechanical damage<br />
does not encircle the needle and usually has<br />
a darkened, wrinkled spot or indentation in<br />
the yellowed area. Around bud break, the<br />
mature, orange telia <strong>for</strong>m on the discolored<br />
spots. Spores are yellow orange.<br />
Another species of spruce needle rust,<br />
Chrysomyxa ledi, has been detected in surrounding<br />
states. The fruiting body of this<br />
fungus resembles white blisters on current<br />
growth and requires an alternate host.<br />
Biology and Life Cycle<br />
This species of spruce needle rust does not<br />
have an alternate host. All necessary spore<br />
stages are found on spruce. During the winter,<br />
the fungus is maturing inside the current<br />
year’s needles and faint yellow bands with<br />
developing orange telia can easily be seen,<br />
particularly on lower branches (Figs. 1 and<br />
2). As bud break approaches, telia become<br />
swollen and contrast with the green or blue<br />
of the needle (Fig. 3).<br />
Figure 1. Early symptoms of<br />
developing rust on Serbian spruce.<br />
Courtesy of Tracey Olson, PDA<br />
Figure 2. Faint yellow and orange<br />
bands with maturing fungus inside<br />
needles. Courtesy of Tracey Olson,<br />
PDA<br />
Figure 3. Swollen telia, or fruiting<br />
bodies (April). Courtesy of Tracey<br />
Olson, PDA<br />
SPRUCE<br />
NEEDLE RUST<br />
Chrysomyxa weirii<br />
(H. S. Jacks)<br />
Yellow bands containing<br />
faint orange fruiting bodies<br />
distinctive of spruce needle<br />
rust. Courtesy of Tracey<br />
Olson, PDA<br />
SPRUCE NEEDLE RUST ........................................................................................................................................................................................................................................................................... ..................................................................... 29 65
Disease Cycle Calendar (Single Year’s Growth of Needles)<br />
May J J A S O N D Jan. F M A M J J A S O N D Jan. F M A M J J A S O N D<br />
Infection<br />
Symptoms<br />
Casting<br />
First Year Second Year Third Year<br />
Bud Break<br />
The heavier the shading, the more intense the infection/symptom/casting.<br />
Figure 4. Spores being released<br />
from the ruptured needle surface.<br />
Courtesy of Tracey Olson,<br />
PDA<br />
Figure 5. Sporulation occurs<br />
during bud break. Courtesy<br />
of Tracey Olson, PDA<br />
At bud break the blisters burst, releasing<br />
massive amounts of yellow-orange spores<br />
(Fig. 4). Wind and splashing rain carry<br />
spores to newly emerging needles on the<br />
same tree or adjacent trees. The infection<br />
period can continue <strong>for</strong> 2–3 weeks after<br />
bud break, and infection rate increases with<br />
high moisture.<br />
The disease cycle starts again with the<br />
newly infected needles harboring the disease<br />
until bud break the following year. Previously<br />
infected needles that have already<br />
dispersed spores will turn rusty brown (Fig.<br />
5), die, and drop from the tree in summer.<br />
Spores will not continue to be produced on<br />
dead needles.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Thoroughly inspect all seedlings be<strong>for</strong>e<br />
planting.<br />
• Space trees to encourage drying.<br />
• Control weeds to reduce the moisture<br />
level around the trees and to increase<br />
coverage if control measures are needed.<br />
• Remove and destroy abandoned hosts<br />
around fi eld to eliminate sources of<br />
inoculum.<br />
Preseason<br />
• Scout trees in winter <strong>for</strong> yellow bands<br />
with orange spots in the center indicating<br />
infection. Scout on overcast day or look at<br />
shaded needles to make it easier to detect<br />
discoloration. Lower branches are often<br />
fi rst to be infected.<br />
• If banding of needles is found, tag several<br />
trees to monitor <strong>for</strong> telia production.<br />
• Scout trees <strong>for</strong> orange telia beginning 1–2<br />
weeks be<strong>for</strong>e bud break and continue until<br />
2–3 weeks after bud break.<br />
• If only a few trees are found with symptoms,<br />
cut and remove them from the fi eld well<br />
be<strong>for</strong>e fruiting bodies mature at bud break.<br />
Growing Season<br />
• Threshold level: No threshold level has<br />
been established <strong>for</strong> this fungus. If the<br />
fungus is present, it can spread rapidly<br />
with favorable environmental conditions.<br />
• This can be a more serious concern <strong>for</strong><br />
<strong>Christmas</strong> tree growers who also dig trees<br />
since it is a disease of regulatory concern<br />
<strong>for</strong> many states. Ask state/regional plant<br />
inspectors about a regulatory threshold <strong>for</strong><br />
this disease.<br />
• Apply a fungicide spray when trees have<br />
broken bud; make subsequent applications<br />
at weekly intervals until needles are<br />
mature or the symptomatic needles have<br />
dropped to the ground (approximately<br />
three to fi ve sprays total).<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Remove and destroy infected trees be<strong>for</strong>e<br />
spore production and release.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Apply a fungicide spray when trees have<br />
broken bud; make subsequent applications<br />
at weekly intervals until needles are<br />
mature or the symptomatic needles have<br />
dropped to the ground (approximately<br />
three to fi ve sprays total).<br />
Next Crop/Prevention<br />
• Do not purchase or accept infected<br />
nursery stock.<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 66
Hosts<br />
• All conifers, especially spruce and Fraser,<br />
Canaan, and balsam fi rs<br />
Damage Potential<br />
• High<br />
Symptoms and Signs<br />
• Small, irregularly shaped yellow spots<br />
(“stippling”) on needles; on fi r, needle<br />
base may be pale<br />
• Rusty or bronzed needles; damage may appear<br />
most severe during hot, dry weather<br />
• Premature needle drop<br />
• Damage heaviest at the bottom inside of<br />
the tree; damaged needles will not recover<br />
from the chlorophyll lost as a result of<br />
mite feeding<br />
• Fine webbing on needles and twigs; cast<br />
skins, dead mites, dirt, and other debris<br />
trapped in the silk<br />
• Infestations frequently occur in pockets,<br />
not distributed evenly through fi eld<br />
Causes of Similar Symptoms<br />
• Rust mites (spruce and fi r)<br />
• Rhizosphaera needle cast (spruce)<br />
• Air pollution<br />
• Aphids (spruce)<br />
Identifi cation<br />
A 15–20X hand lens or small microscope<br />
is required to view spider mites and eggs.<br />
Adult spruce spider mites are oval and only<br />
1 ⁄50 inch (0.5 mm) long. They have thin<br />
hairs, or setae, on the top of their convex<br />
bodies. The body color depends on the host<br />
and varies from pale green to dark green<br />
or dark red; legs are generally pale in color.<br />
The fi rst stage, a six-legged larva, is salmon<br />
colored until it has fed <strong>for</strong> a short time.<br />
After feeding, the larva and all subsequent<br />
stages are generally green or dark red. The<br />
eggs are rounded and vary from tan (active<br />
season eggs) to red (overwintering eggs).<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Each egg has a single hairlike stripe on the<br />
top, which can be used to distinguish spruce<br />
spider mite eggs from other spider mite eggs<br />
that may be found on conifers.<br />
Two other spider mites occur on <strong>Christmas</strong><br />
trees: two-spotted spider mite and<br />
admes spider mite. The two-spotted spider<br />
mite is generally green with two dark spots,<br />
one on either side of the oval body. It is<br />
slightly smaller than spruce spider mite and<br />
overwinters as an orange female. The admes<br />
spider mite has only been found on spruce<br />
and is larger than spruce spider mite. The<br />
body is more fl attened and reddish brown<br />
with long, pale legs. The admes mite overwinters<br />
as an egg.<br />
Biology and Life Cycle<br />
Spruce spider mites can develop from an egg<br />
to an adult in 2–3 weeks under normal conditions<br />
(Figs. 1 and 2). It is a cool-season mite<br />
and attains the highest populations in spring<br />
and fall; temperatures above 80–90°F will<br />
result in population decline. During the hot,<br />
dry weather in summer, spruce spider mites<br />
seem to disappear, only to reappear when<br />
temperatures moderate in late summer or fall.<br />
Spider mites are still present during summer,<br />
but feeding and reproduction are greatly<br />
reduced. Predatory mites are very common<br />
on the host plants at this time of year.<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
Monitor <strong>for</strong> egg hatch.<br />
Apply dormant oil.<br />
Figure 1. Adult<br />
spruce spider<br />
mite. Courtesy<br />
of Rayanne D.<br />
Lehman, PDA<br />
Figure 2. Adult<br />
spruce spider<br />
mite. Courtesy<br />
of Rayanne D.<br />
Lehman, PDA<br />
SPRUCE<br />
SPIDER MITE<br />
Oligonychus ununguis<br />
(Jacobi)<br />
Spruce spider mite feeding<br />
damage. Courtesy of<br />
Eric R. Day, Virginia Tech,<br />
Bugwood.org (#0717020)<br />
SPRUCE SPIDER MITE ............................................................................................................................................................................................................................................................................ ..................................................................... 29 67
Figure 3. Overwintering spruce<br />
spider mite eggs. Courtesy of<br />
Sandy Gardosik, PDA<br />
Figure 4. Newly molted spruce<br />
spider mite adults. Courtesy of<br />
Sandy Gardosik, PDA<br />
Figure 5. Chlorotic spots resulting<br />
from spider mite feeding.<br />
Courtesy of PDA<br />
Figure 6. Spider mite feeding<br />
damage on older growth.<br />
Courtesy of USDA Forest Service<br />
Northeastern Area Archive,<br />
Bugwood.org (#1396124)<br />
Spruce spider mites follow the typical<br />
spider mite life cycle: egg, six-legged larva,<br />
two eight-legged nymphal stages, and adult<br />
(male or female) (Figs. 3 and 4). Between<br />
each of the active stages, a resting stage,<br />
or chrysalis, is <strong>for</strong>med as the mite prepares<br />
to shed its outer skin. This molting stage is<br />
generally not affected by chemical controls.<br />
The male completes development fi rst and<br />
may be seen “guarding” the female chrysalis<br />
until she emerges and mating occurs. Mated<br />
females produce both male and female offspring,<br />
while unmated females produce only<br />
females. Depending on temperature, each<br />
female can deposit an average of 30–40 eggs<br />
during her lifetime. After the fi rst generation,<br />
overlap of generations is common and<br />
all stages are present at any given time.<br />
Spider mites have piercing-sucking<br />
mouthparts. As they feed, they withdraw<br />
sap containing chlorophyll from the needles<br />
(Fig. 5). They prefer to feed on older growth<br />
and will generally not feed on current-year<br />
needles until they have hardened off (Fig.<br />
6). This species generally does not produce<br />
copious amounts of silk. But, when populations<br />
are high, larvae can spin down on the<br />
silk threads they produce and be carried by<br />
air currents, insects, birds, or other animals<br />
to new host plants (Fig. 7). Six or more<br />
generations occur a year in Pennsylvania.<br />
Figure 7. Spider mite webbing buildup<br />
during a more severe infestation. Courtesy<br />
of USDA Forest Service Region 4 Archive,<br />
Bugwood.org (#0949037)<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• During site selection, consider elevation<br />
and aspect of the fi eld. Warmer temperatures<br />
generally associated with southfacing<br />
slopes and lower elevations favor<br />
spruce spider mite populations.<br />
• Drought-prone locations favor population<br />
buildup.<br />
• Inspect nursery stock <strong>for</strong> infestations.<br />
Preseason<br />
• Manage groundcover and surrounding<br />
area to encourage natural predators.<br />
• Growing degree days: Based on observations<br />
in Pennsylvania, egg hatch occurs at<br />
50–121 GDDs.<br />
• Scout trees <strong>for</strong> overwintering eggs in<br />
very early spring with a 15X hand lens.<br />
Examine shoots <strong>for</strong> red, rounded eggs usually<br />
located on twigs at the base of needles<br />
or around buds. Monitor <strong>for</strong> egg hatch<br />
by tagging one or more trees known to<br />
have a population of overwintering eggs.<br />
Hatch will generally begin on the south<br />
side of the tree. Make observations every<br />
few days to determine when eggs begin<br />
to hatch. Egg hatch occurs prior to bud<br />
break. Record percentage of egg hatch if<br />
miticide use is planned.<br />
• Scout <strong>for</strong> active <strong>for</strong>ms by holding a<br />
light-colored surface (paper, paper plate,<br />
painted clipboard, inside back cover of<br />
this manual, etc.) beneath a branch. Tap<br />
the branch sharply by hand or with a stick<br />
(Fig. 8). Mites will be dislodged and drop<br />
to the monitoring surface. Tilt the surface<br />
slightly to allow dirt and debris to slide off<br />
and then observe <strong>for</strong> mites. Spider mites<br />
will begin to crawl and appear as minute<br />
dark dots moving slowly over the surface.<br />
Examine with your hand lens to verify<br />
that they are spider mites. Sample several<br />
branches around the tree and multiple<br />
trees per block to determine population<br />
level.<br />
• Smearing suspected mites can be another<br />
test—dark green or dark red smears on the<br />
monitoring surface usually indicate spruce<br />
spider mites.<br />
• Populations can be estimated by examining<br />
shoots of current growth taken from<br />
the bottom third of trees. Use a hand lens<br />
to check <strong>for</strong> active <strong>for</strong>ms or viable eggs.<br />
Sample about 15 shoots per acre or a minimum<br />
of 10 shoots in a small block; sample<br />
every part of block as populations tend to<br />
NEEDLE DISCOLORATION AND INJURY ................................................................................................................................................................................................................................................ 68
e spotty in a fi eld. Determine the percentage<br />
of shoots that have mites or mite<br />
eggs. Repeat this procedure every several<br />
weeks to detect population fl uctuations.<br />
• Emerald green arborvitae is a good indicator<br />
plant and will show signs of infestation<br />
early. If planted, monitor closely <strong>for</strong> fi rst<br />
signs of spruce spider mites.<br />
Growing Season<br />
• Continue monitoring populations using<br />
the beating method or by observing eggs<br />
or active mites on branches. Repeat<br />
sampling every 3–4 weeks throughout the<br />
growing season and within one week following<br />
pesticide application.<br />
• Watch <strong>for</strong> increase in population numbers,<br />
which may require control. Damage<br />
observations, although helpful in locating<br />
populations, are not accurate measures of<br />
population levels.<br />
• Drought and/or warm weather around<br />
autumn could delay fall egg hatch.<br />
• Growing degree days: Peak mite activity<br />
occurs at 192–363 and 2,375–2,806<br />
GDDs.<br />
• Threshold level—two methods described<br />
below determine the economic threshold:<br />
— Mites per branch: More than 10 mites<br />
per branch on most branches sampled<br />
indicate that pesticide intervention is<br />
recommended.<br />
— Percentage of infested trees: Divide<br />
the number of shoots with mites and/or<br />
mite eggs by the total number of shoots<br />
examined and multiply by 100 to<br />
calculate the percentage. Refer to the<br />
table below to determine if pesticide<br />
intervention is recommended.<br />
THRESHOLD LEVEL<br />
Economic<br />
Size of <strong>Tree</strong><br />
Threshold*<br />
Less than waist high<br />
(< 3.2 feet) Up to 40%<br />
Waist high (3.2 feet),<br />
year be<strong>for</strong>e sale Up to 20%<br />
Greater than waist<br />
high (> 3.2 feet),<br />
year of sale Up to 10%<br />
*Threshold levels may be different <strong>for</strong> trees<br />
that will be dug as opposed to cut. Check<br />
with your state/regional plant inspector <strong>for</strong><br />
acceptable mite levels.<br />
• At the end of the season, update records<br />
and evaluate results.<br />
Control Options<br />
Biological<br />
• While scouting <strong>for</strong> spruce spider mites,<br />
also look <strong>for</strong> natural predators such as<br />
fast-moving, pale phytoseiid mites, hover<br />
fl y larvae, lacewing larvae, dusty wings,<br />
and lady beetles. All are effective predators<br />
and may help control the population<br />
of spruce spider mites. Refer to Appendix<br />
B: Biological Controls Photo Chart <strong>for</strong><br />
pictures.<br />
Mechanical<br />
• Streams of water under pressure can effectively<br />
wash mites from trees. However, this<br />
is not practical in the fi eld situation, but<br />
long periods of heavy rain can signifi cantly<br />
reduce mite populations.<br />
Biorational<br />
• Dormant oil applied prior to egg hatch<br />
can reduce the population. Note: Oil will<br />
remove the blue coloring from spruce.<br />
Chemical<br />
• General insecticides are not as effective<br />
<strong>for</strong> control as are specifi c chemicals referred<br />
to as miticides. General insecticides<br />
are often detrimental to populations of<br />
benefi cial insects and mites.<br />
• Numerous miticides are available <strong>for</strong> controlling<br />
spruce spider mite on <strong>Christmas</strong><br />
trees. Ovicides that target eggs and materials<br />
that control both eggs and active <strong>for</strong>ms<br />
often provide the longest control. Ovicides<br />
generally do not work on overwintering<br />
eggs. For best results, apply controls when<br />
population is low or starting to build.<br />
Evaluate control after 5 days.<br />
• A second application may be needed in<br />
7–10 days, unless prohibited on the label.<br />
Some labels recommend application one<br />
time per year or even every other year to<br />
lessen chance of resistance developing in<br />
the population.<br />
• Spider mites are capable of quickly becoming<br />
resistant to a particular miticide<br />
due to their rapid reproduction. To avoid<br />
resistance, alternate classes of chemicals<br />
every third application.<br />
Next Crop/Prevention<br />
• Purchase and plant pest-free nursery stock<br />
from a reputable company.<br />
Figure 8. Tapping branches over<br />
white paper to dislodge mites.<br />
Courtesy of Brian Schildt, PDA<br />
SPRUCE SPIDER MITE ............................................................................................................................................................................................................................................................................. 69
SWISS<br />
NEEDLE CAST<br />
Phaeocryptopus<br />
gäumannii<br />
(T. Rohde) Petr.<br />
Foliage with symptoms and<br />
signs of Swiss needle cast<br />
infection. Courtesy of Tracey<br />
Olson, PDA<br />
Host<br />
• Douglas-fi r<br />
Damage Potential<br />
• Moderate–high<br />
Symptoms and Signs<br />
• May fi rst develop on lower branches<br />
Spring Through Fall<br />
• Two parallel rows of tiny, black fruiting<br />
bodies (pseudothecia) on the underside<br />
of fi rst- , second- , or third-year needles<br />
• Fruiting bodies may not develop on<br />
current-year infected needles until after<br />
a frost<br />
• Dieback of needle tips resembling drought<br />
damage<br />
• Yellowing or mottling of previously<br />
infected needles<br />
• Browning and casting of needles infected<br />
at least one year prior; heaviest casting<br />
seen on interior of lower branches<br />
Causes of Similar Symptoms<br />
• Rhabdocline needle cast<br />
• Flyspeck<br />
• Cooley spruce gall adelgid<br />
• Environmental stresses<br />
• Nutrient imbalances<br />
• Winter burn<br />
Identifi cation<br />
Symptoms are present on needles within<br />
3 years of infection. The fruiting bodies of<br />
Swiss needle cast are easily detected and<br />
can be seen with a hand lens anytime<br />
throughout the year. Look <strong>for</strong> two bands of<br />
tiny, black structures ( 1 ⁄240 inch; 0.1 mm)<br />
arising from the stomates on the undersides<br />
of mottled or brown-tipped needles.<br />
Figure 1. Black fruiting bodies on the undersides<br />
of needles pushing up through the<br />
stomates. Courtesy of Tracey Olson, PDA<br />
Occasionally, a purple band will be visible<br />
between the dead tip and green base of the<br />
needle. These fruiting structures may occur<br />
on all ages of needles—even on needles<br />
that appear healthy—so it is important to<br />
examine many needles on a tree, not just<br />
symptomatic current-year needles. Fruiting<br />
bodies found on older needles attached<br />
to the tree are still capable of releasing<br />
spores. Infected trees may appear thin and,<br />
in severe cases, may retain only previous<br />
year’s growth. Pay particular attention to<br />
lower and inner branches when scouting <strong>for</strong><br />
this disease. Un<strong>for</strong>tunately, by the time this<br />
disease is found, much of the green foliage<br />
on the tree is infected. Rhabdocline and<br />
Swiss needle casts can both be found on the<br />
same tree.<br />
Biology and Life Cycle<br />
Like most other fungal diseases, Swiss<br />
needle cast favors rainy, moist, and cool<br />
weather. Fruiting bodies develop through<br />
the stomata on the underside of the needles<br />
by early winter and are visible from late<br />
winter into spring, but they only mature<br />
about the time of bud break (Figs. 1 and 2).<br />
Fruiting bodies from 1- to 3-year-old needles<br />
are capable of producing spores, which are<br />
released from the undersides of needles and<br />
carried by splashing water or wind to new<br />
growth, where they penetrate and infect the<br />
needle (Fig. 3). Though primary infection<br />
occurs on new growth, occasionally previous<br />
years’ needles will also become infected at<br />
this time (Fig. 4). This signifi cantly increases<br />
the infection potential of this disease.<br />
Symptoms are much slower to develop and<br />
may not be apparent <strong>for</strong> as long as 2 years<br />
after infection. Spore release continues <strong>for</strong><br />
a longer time than Rhabdocline, the other<br />
common needle cast disease of Douglas-fi r.<br />
Figure 2. Fruiting bodies arranged in two rows<br />
on the undersides of needles. Courtesy of<br />
Tracey Olson, PDA<br />
NEEDLE DISCOLORATION AND INJURY .......... ................................................................................................................................................................................................................................................ 70
Disease Cycle Calendar (Single Year’s Growth of Needles)<br />
May J J A S O N D Jan. F M A M J J A S O N D Jan. F M A M J J A S O N D<br />
Infection<br />
Symptoms<br />
Casting<br />
First Year Second Year Third Year<br />
Bud Break<br />
The heavier the shading, the more intense the infection/symptom/casting.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant trees with expanded spacing (6 feet<br />
by 6 feet, or 1.83 by 1.83 m) to allow <strong>for</strong><br />
good air circulation. Avoid planting on a<br />
north-facing slope, near hardwood <strong>for</strong>ests,<br />
or in a low, damp area. These types of<br />
areas generally retain moisture on needles<br />
<strong>for</strong> longer period of time.<br />
• Plant resistant or tolerant tree varieties;<br />
avoid Douglas-fi rs from Rocky Mountain<br />
seed sources.<br />
• Remove and destroy unmanaged Douglasfi<br />
r that may act as a source of inoculum.<br />
Preseason<br />
• Properly water and fertilize to promote<br />
good health of trees.<br />
• Maintain proper weed control year-round.<br />
• Begin scouting <strong>for</strong> fruiting bodies in 4- to<br />
10-year-old trees in late winter. Select 20<br />
or more trees and examine the undersides<br />
of current and older years’ needles on<br />
three branches per tree. Select branches<br />
from the lower third of the tree and look<br />
at 1- and 2-year-old needles. Pay particular<br />
attention to trees that appear off color<br />
or thin. Concentrate scouting on trees<br />
planted in areas that are more conducive<br />
<strong>for</strong> disease development.<br />
• Scouting can be more effective on an<br />
overcast day when needle mottling is<br />
more apparent.<br />
Growing Season<br />
• Threshold level: Ask state/regional plant<br />
inspectors about regulatory thresholds.<br />
• In late April/early May, begin regular<br />
scouting <strong>for</strong> bud break in each target<br />
block. Make the fi rst fungicide application<br />
when the buds are ½–2 inches (1.27–5.08<br />
cm).<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Minimize disease transmission by shearing<br />
only when trees are dry.<br />
• Shear healthy trees/blocks fi rst to minimize<br />
transmission of spores on equipment<br />
and personnel.<br />
• Sterilize equipment in contact with the<br />
disease by soaking in denatured alcohol<br />
<strong>for</strong> 3 minutes.<br />
• Remove and destroy heavily infected trees<br />
prior to bud break.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• If treatment is necessary, apply fungicide<br />
when shoots are ½–2 inches (1.27–5.08<br />
cm). Make a second application 2–3<br />
weeks later. A third application may be<br />
necessary if rainfall is high and temperatures<br />
remain cool. These treatments<br />
coincide with the second and third applications<br />
<strong>for</strong> Rhabdocline needle cast.<br />
• Since infected needles may remain<br />
attached to the tree and continue to<br />
produce spores <strong>for</strong> up to 3 years, chemical<br />
control <strong>for</strong> 3 consecutive years may be<br />
necessary.<br />
• If Rhabdocline needle cast is also present,<br />
make sure to begin fungicide applications<br />
at fi rst sign of bud break.<br />
Next Crop/Prevention<br />
• Inspect plants/nursery stock; buy from a<br />
reputable company.<br />
• Collect seeds from trees showing<br />
resistance <strong>for</strong> a seed-collection program.<br />
Figure 3. Infected needles<br />
brown from tip down. Courtesy<br />
of Brian Schildt, PDA<br />
Figure 4. Previous seasons’<br />
infected needles may be cast<br />
or remain on the tree. Courtesy<br />
of USDA Forest Service North<br />
Central Research Station<br />
Archive, Bugwood.org<br />
(#1406194)<br />
SWISS NEEDLE CAST .............................................................................................................................................................................................................................................................................. 71
SHOOT AND BRANCH INJURY<br />
Diseases, insects, and mite pests that kill<br />
or cause serious damage to new growth<br />
and entire branches are discussed on<br />
pages 73–104. The damage may distort<br />
or kill the shoot or branch but usually<br />
does not result in death of the entire<br />
tree.<br />
SHOOT AND BRANCH INJURY .............................................................................................................................................................................................................................................................. 72
Hosts<br />
• Pines: Austrian, Scotch, eastern white,<br />
Jack, red, lodgepole, Ponderosa, Virginia,<br />
and others<br />
Damage Potential<br />
• Moderate–severe<br />
Symptoms and Signs<br />
• Small, elliptical cankers at bases of<br />
needles<br />
• Resin droplets at canker site<br />
• Clusters of small, black fruiting bodies<br />
on cankered tissue<br />
• Flagged (dead and brown) branches<br />
throughout tree<br />
• Sapwood beneath canker stained gray<br />
to black<br />
Causes of Similar Symptoms<br />
• Frost damage<br />
• Pales weevil adult feeding<br />
• Diplodia (Sphaeropsis) tip blight<br />
• White pine blister rust<br />
Identifi cation<br />
Four native North America species of<br />
Atropellis cause canker on pines. Atropellis<br />
piniphila and A. pinicola occur in the western<br />
and northwestern parts of the country,<br />
respectively. Atropellis apiculata is only found<br />
in Virginia and North Carolina. The species<br />
occurring in Pennsylvania is A. tingens. This<br />
article focuses on A. tingens.<br />
This fungal disease of pines causes small,<br />
elliptical, blue-black cankers (or areas of<br />
dead tissue) about 0.79 inch (2 cm) long<br />
underneath the bark of twigs and branches,<br />
originating at needle bases. Small resin<br />
droplets <strong>for</strong>m on the bark surface around<br />
the margins of cankers. Multiple cankers<br />
may join to girdle a twig or branch. Needles<br />
on these girdled twigs/branches begin to discolor<br />
and the twig/branch eventually dies.<br />
Figure 1. Fruiting structures of Atropellis canker<br />
on an infected Scotch pine branch. Courtesy of<br />
Tracey Olson, PDA<br />
These fl agged branches are most noticeable<br />
in spring and early summer. In addition<br />
to patches of dead branches, this disease<br />
can also be recognized by the clusters of<br />
black, cup-shaped fruiting bodies that are<br />
0.08–0.16 inch (2–4 mm) long and arise on<br />
the dead bark of 2- to 3-year-old cankers.<br />
Cutting into cankered areas reveals darkly<br />
stained sapwood. This distinguishes Atropellis<br />
canker from Diplodia, which discolors<br />
the wood reddish brown.<br />
The death of branches due to A. tingens<br />
can disfi gure <strong>Christmas</strong> trees, making them<br />
less economically desirable.<br />
Biology and Life Cycle<br />
Approximately one year after infection<br />
begins, the fungus develops barely visible,<br />
round, black structures (stromata) on the<br />
surface of the bark above the cankers. These<br />
structures produce noninfectious spores.<br />
Black, cup-shaped fruiting bodies (apothecia)<br />
<strong>for</strong>m on the bark surface (Fig. 1) approximately<br />
2–3 years after infection. Apothecia<br />
are 0.08–0.16 inch (2–4 mm) in diameter<br />
and can be seen easily without magnifi cation.<br />
Beginning in summer, these fruiting bodies<br />
swell in moist weather conditions and <strong>for</strong>cefully<br />
release infectious spores. Spore release<br />
continues through the early fall.<br />
The fruiting bodies continue to sporulate<br />
annually, and will even do so <strong>for</strong> a short<br />
time on a standing dead or fallen tree if it<br />
remains wet. The spores are dispersed by<br />
wind and may be carried up to 328 feet<br />
(100 m) from the host plant. Spores that<br />
come in contact with healthy bark or needle<br />
scars germinate under moist conditions. The<br />
fungus enters the tree through the base of<br />
the needle. Infection occurs in living tissue<br />
and grows rapidly within the fi rst year or<br />
two, staining the wood tissue blue black as<br />
it grows (Fig. 2). Fungus growth slows after<br />
the fi rst couple of years; after 10 years, it<br />
becomes inactive.<br />
Figure 2. Distinctive blue-black stain is<br />
characteristic of Atropellis infection. Courtesy<br />
of Tracey Olson, PDA<br />
ATROPELLIS<br />
CANKER<br />
Atropellis tingens<br />
Lohman and Cash<br />
(also A. apiculata<br />
Lohman, Cash, and<br />
R. W. Davidson,<br />
A. pinicola Zeller<br />
and Goodd, and<br />
A. piniphila [Weir]<br />
Lohman and Cash)<br />
Flagging and girdled<br />
branches symptomatic of<br />
Atropellis canker.<br />
Courtesy of Tracey Olson,<br />
PDA<br />
ATROPELLIS CANKER ............................................................................................................................................................................................................................................................................. ..................................................................... 71 73
Disease Cycle Calendar (Single Year’s Growth of Needles)<br />
May J J A S O N D Jan. F M A M J J A S O N D Jan. F M A M J J A S O N D<br />
Infection<br />
Symptoms<br />
Casting<br />
First Year Second Year Third Year<br />
Bud Break<br />
The heavier the shading, the more intense the infection/symptom/casting.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant trees with enough space between<br />
them to encourage airfl ow.<br />
• Remove and destroy any overgrown<br />
pines surrounding the proposed block to<br />
decrease chance of infection.<br />
Preseason<br />
• Maintain plant vigor and health with<br />
proper fertilization.<br />
• Control weeds under and around trees<br />
throughout the year.<br />
• Scout trees <strong>for</strong> fl agged branches and apothecia<br />
on the bark of infected branches.<br />
• Immediately prune and destroy any<br />
infected material found. Remove and<br />
destroy any tree with multiple cankers.<br />
Growing Season<br />
• Continue to scout throughout the year<br />
and prune out and burn infected material.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Prune cankered branches 6–12 inches<br />
(15.2–30.5 cm) below the canker or<br />
where the branch attaches to the main<br />
stem. Remove and burn infected material.<br />
Disinfect shears with 70 percent alcohol<br />
or a bleach solution between cuts, as<br />
spores can be spread on tools.<br />
• Remove and burn trees with cankers<br />
on the main stem or trees with a heavy<br />
infection.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• No recommendations are available<br />
at this time.<br />
Next Crop/Prevention<br />
• Buy and plant disease-free stock only.<br />
SHOOT AND BRANCH INJURY .............................................................................................................................................................................................................................................................. 74
Hosts<br />
• All true fi rs<br />
Damage Potential<br />
• High<br />
Symptoms<br />
• Flat top or crooked terminal<br />
• Gouting (swelling) around buds and<br />
internodes<br />
• Stiff, infl exible trunk and large lateral<br />
branches<br />
• White, cottony masses on trunk and<br />
large branches<br />
• Dead shoots or branches (red or brown<br />
needles)<br />
Causes of Similar Symptoms<br />
• Phytophthora root rot<br />
Identifi cation<br />
Balsam woolly adelgid females are softbodied,<br />
spherical, purplish-black, wingless<br />
insects. They are about 1 ⁄25 inch long (< 1<br />
mm) and are not mobile. During the winter,<br />
immature nymphs can be found on bark.<br />
They are dark and have white, waxy rods<br />
down their backs and around the edges<br />
of their bodies. At this stage, they closely<br />
resemble the eggs of balsam twig aphid. As<br />
the mature, they continue to secrete this<br />
waxy substance, which gives them a covering<br />
that may cause them to resemble minute<br />
cotton balls by the time females are present.<br />
Eggs are oblong and deposited in a cluster<br />
behind the female. The orange-brown<br />
crawlers are the only stage with functional<br />
legs. Red eyespots are also visible on the<br />
crawlers. A hand lens is needed to effectively<br />
locate all stages.<br />
Symptoms can be helpful in detecting<br />
balsam woolly adelgid, but it may take several<br />
months <strong>for</strong> damage to become visible.<br />
Symptoms develop slowly since the insect<br />
only feeds on the bark, not the needles.<br />
When a tree begins to show symptoms,<br />
it means that the tree has been infected<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
since be<strong>for</strong>e bud break of that growing<br />
season. The fi rst most noticeable symptom<br />
is a fl at top or weak, crooked terminal. As<br />
the infestation progresses, trees may have<br />
swelling, or gouting, around the buds and<br />
shoot nodes. The swollen growth is a result<br />
of a chemical inserted as the adelgid feeds<br />
on the branches. When adelgids feed on the<br />
main trunk, they cause the tree to become<br />
brittle due to the <strong>for</strong>mation of scar tissue.<br />
When harvesting, examine stumps <strong>for</strong> this<br />
dark ring of wood.<br />
Biology and Life Cycle<br />
No male balsam woolly adelgids are present<br />
in North America. Females reproduce<br />
parthenogenetically (by producing eggs<br />
that are exact clones of the adult). The<br />
immature nymphs overwinter on the trunk,<br />
larger branches, and around buds. In early<br />
spring, they mature and the female adelgids<br />
are present. Females cover themselves<br />
with waxy, wool-like covering—hence<br />
the name balsam woolly adelgid (Fig. 1).<br />
Figure 1. Adelgids covered with a protective<br />
white, woolly wax. Courtesy of USDA Forest<br />
Service Region 8 Archive, Bugwood.org<br />
(#1510053)<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
Apply horicultural oil.<br />
BALSAM<br />
WOOLLY<br />
ADELGID<br />
Adelges piceae<br />
(Ratzeburg)<br />
Swelling, or gouting, on<br />
branches resulting from<br />
abnormal cell growth<br />
caused by adelgid chemical<br />
secretion. Courtesy of USDA<br />
Forest Service Region 8<br />
Archive, Bugwood.org<br />
(#1510051)<br />
BALSAM WOOLLY ADELGID ................................................................................................................................................................................................................................................................. ..................................................................... 71 75
Figure 2. Cluster of eggs produced<br />
by females under white,<br />
woolly wax. Courtesy of Scott<br />
Tunnock, USDA Forest Service,<br />
Bugwood.org (#2252066b)<br />
Each female produces a cluster of eggs<br />
under the woolly mass surrounding her body<br />
(Fig. 2). Up to 200 eggs can be produced in<br />
the laboratory, but in the fi eld the number<br />
of eggs is reduced and depends on the condition<br />
of the host tree. Eggs hatch in about a<br />
month, around the time of bud break.<br />
When crawlers emerge, they can live<br />
up to 2 days while searching <strong>for</strong> a place<br />
to settle. Since crawlers lack mouthparts,<br />
they must molt be<strong>for</strong>e feeding can begin.<br />
These nymphs insert their piercing-sucking<br />
mouthparts into the bark to feed, but, once<br />
inserted, the adelgid is not able to move.<br />
While feeding, adelgids secrete a chemical<br />
that causes the abnormal growth of cells in<br />
the area. This leads to swelling or gouting,<br />
branch dieback, and possible death of the<br />
tree (Fig. 3). The stationary crawlers will<br />
molt several times be<strong>for</strong>e becoming adults.<br />
In midsummer, these adults lay eggs, which<br />
again hatch into crawlers. The crawlers<br />
overwinter and start the cycle again the following<br />
spring (Fig. 4). In Pennsylvania, two<br />
generations occur per year.<br />
Figure 3. Gouting, or swelling, from growth<br />
of cells in the area. Courtesy of Robert L.<br />
Anderson, USDA Forest Service, Bugwood.org<br />
(#1748038)<br />
Figure 4. Overwintering balsam woolly adelgid<br />
nymphs. Courtesy of Rayanne D. Lehman, PDA<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Remove and destroy unmanaged fi r that<br />
may act as a source of inoculum.<br />
• Purchase and plant pest-free stock from a<br />
reputable nursery.<br />
Preseason<br />
• Scout at waist height <strong>for</strong> adelgid symptoms<br />
on branches (swollen areas) and<br />
main trunk (white, cottony masses). Look<br />
<strong>for</strong> fl at tops on trees or dark reddish rings<br />
in wood of cut stumps.<br />
Growing Season<br />
• Maintain good weed control.<br />
• Butt-prune trees when appropriate.<br />
• Scout <strong>for</strong> adult adelgids in late spring<br />
through late summer. Look <strong>for</strong> the white,<br />
waxy, wool-like covering, remove suspected<br />
area, and examine it with a hand lens<br />
or under a microscope to confi rm adelgid<br />
infestation.<br />
• Tag any infested or suspected trees.<br />
• Scout <strong>for</strong> terminal damage in fall after tree<br />
growth has ended <strong>for</strong> the season. Look<br />
<strong>for</strong> fl at tops by walking back and <strong>for</strong>th<br />
through rows spaced 6–10 feet (1.83–3.05<br />
m) apart. If a suspect tree is found, rock<br />
the tree back and <strong>for</strong>th to check <strong>for</strong> a stiff<br />
trunk. If not confi rmed, fl ag the tree and<br />
recheck in about a month.<br />
• If the adelgid is found, treat the fi eld<br />
be<strong>for</strong>e bud break of the following season.<br />
Treat after harvesting any trees from<br />
fi eld and possibly time with other pest<br />
treatments (e.g., with balsam twig aphid<br />
control).<br />
• Examine stumps from the current season<br />
<strong>for</strong> evidence of scar tissue.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
SHOOT AND BRANCH INJURY .............................................................................................................................................................................................................................................................. 76
Control Options<br />
Biological<br />
• Several <strong>for</strong>eign predators have been introduced,<br />
although the level of control they<br />
provide is still unclear. Aphidecta obliterate<br />
(L.), Laricobius erichsonii (Rosenhauer),<br />
and Pullus impexus (Mulsant) are beetles.<br />
Aphidoletes thompsoni Mohn, Cremifania<br />
nigrocellulata (Czerny), and Leucopis obscura<br />
Haliday are fl ies.<br />
Mechanical<br />
• Cut and burn heavily infested trees that<br />
will not be salable. Do not cut during<br />
crawler activity.<br />
• Clear-cut infested blocks.<br />
Biorational<br />
• Horticultural oil spray: apply thoroughly<br />
to the trunk and bark of large branches<br />
in late fall, winter, or early spring; oil can<br />
damage foliage if not applied properly.<br />
— Only apply oil when temperatures are<br />
above freezing.<br />
— Oil will remove “bloom,” or blue color,<br />
from blue specimens.<br />
Chemical<br />
• Use a high-pressure sprayer to apply an<br />
appropriate insecticide during the fi rstgeneration-crawler<br />
stage; fully cover all<br />
trees to achieve the best control. Note:<br />
Control <strong>for</strong> balsam woolly adelgid may<br />
kill natural predators of other pests.<br />
• Scout one month after treatment to make<br />
sure adelgids are dead; look <strong>for</strong> new woolly<br />
spots.<br />
Next Crop/Prevention<br />
• Scout every year to determine if and when<br />
treatment or re-treatment is necessary.<br />
BALSAM WOOLLY ADELGID .................................................................................................................................................................................................................................................................. 77
BOTRYTIS<br />
BLIGHT<br />
Botrytis cinerea<br />
Pers.: Fr.<br />
New growth infected with<br />
Botrytis blight. Courtesy of<br />
Tracey Olson, PDA<br />
Hosts<br />
• All <strong>Christmas</strong> tree species<br />
Damage Potential<br />
• Low<br />
Symptoms and Signs<br />
• Spots on needles and shoots that appear<br />
water soaked<br />
• Blighted new growth that resembles frost<br />
damage<br />
• Brown lesions on needles and shoots that<br />
can grow and girdle the shoots<br />
• Gray, fuzzy, fungus structure on infected<br />
shoots and needles<br />
• When the fungus is dry, clouds of spores<br />
will be released when disturbed<br />
Causes of Similar Symptoms<br />
• Atropellis canker<br />
• Diplodia (Sphaeropsis) tip blight<br />
• Cytospora canker<br />
• Frost damage<br />
• Pales weevil adult feeding<br />
Identifi cation<br />
This fungal disease can infect hundreds of<br />
woody and herbaceous plants, including most<br />
species of trees grown as <strong>Christmas</strong> trees.<br />
This fungal disease does not produce typical<br />
fruiting structures as seen in other conifer diseases.<br />
Instead, Botrytis cinerea, or gray mold,<br />
produces a gray, weblike mycelium over the<br />
surface of the diseased area. Spores are borne<br />
on stalks on this hairlike growth.<br />
Symptoms appear in spring and may<br />
follow frost damage or an extended period of<br />
shoot elongation due to cool temperatures.<br />
Infection will not occur on woody tissue,<br />
but the succulent new growth is susceptible.<br />
Infected tissue may appear water soaked<br />
and will eventually turn brown (Fig. 1).<br />
Figure 1. New shoot browned from Botrytis<br />
infection. Courtesy of Tracey Olson, PDA<br />
Figure 2. Dieback of new growth caused by<br />
Botrytis. Courtesy of Rayanne D. Lehman, PDA<br />
Tip dieback may occur when the infected<br />
areas girdle the shoots (Fig. 2). The disease<br />
develops under humid conditions with cool<br />
temperatures. Seedling beds are generally<br />
most susceptible to this blight because of<br />
the tight spacing.<br />
Biology and Life Cycle<br />
Botrytis overwinters in the Northeast in<br />
moist, decaying plant tissue either as mycelium<br />
or as small, black, resting structures,<br />
or sclerotia. As the temperatures warm in<br />
spring, these resting structures start to germinate<br />
and threadlike mycelium will begin<br />
to cover the diseased tissue (Fig. 3). Massive<br />
amounts of spores are <strong>for</strong>med on this<br />
mycelium, especially in the morning hours.<br />
They are spread to healthy tissue by wind<br />
or splashing water. When the spores come<br />
in contact with new plant tissue and during<br />
periods of high humidity or moisture, they<br />
will begin to germinate and penetrate the<br />
plant tissue. Spores can be produced continuously<br />
during moist conditions and may<br />
remain dormant <strong>for</strong> up to 3 weeks be<strong>for</strong>e<br />
they germinate.<br />
Figure 3. Threadlike mycelium with spores<br />
covering infected tissue. Courtesy of<br />
Rayanne D. Lehman, PDA<br />
SHOOT AND BRANCH INJURY ........................<br />
.............................................................................................................................................................................................................................................................. 78
This fungus can persist in either dead or<br />
living plant tissue. Infection typically takes<br />
place in shaded areas with high humidity,<br />
such as where trees are planted close<br />
together and on the lower branches of<br />
trees. A growing season that has an early<br />
warm spell, causing bud and shoot growth<br />
to begin, followed by an extended cool/<br />
damp period, which suspends needle and<br />
shoot development, is especially favorable<br />
<strong>for</strong> Botrytis. This fungus is a relatively weak<br />
pathogen. New shoots that are injured by<br />
frost or mechanical means are most prone to<br />
this disease.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Space trees to encourage drying.<br />
• Control weeds to reduce the moisture<br />
level around the trees.<br />
Preseason<br />
• Promote tree health by using good cultural<br />
practices, such as proper fertilization<br />
and adequate watering.<br />
Growing Season<br />
• Scout trees that are densely planted and<br />
shaded. Look <strong>for</strong> tree shoots that appear<br />
rain soaked or exhibit the gray webbing.<br />
• Prevent tree injury from machinery.<br />
• Control damage from insects and other<br />
diseases.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Prune infected tips out of established<br />
trees after foliage has died. Disinfect<br />
shears with 70 percent alcohol or a bleach<br />
solution between cuts since spores can be<br />
spread on tools.<br />
• Remove infected seedlings from seedling<br />
beds to prevent disease spread.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• In general, fungicide sprays are unnecessary<br />
<strong>for</strong> established <strong>Christmas</strong> tree plantings.<br />
• For seedling beds, preventative fungicide<br />
sprays may be helpful in preventing infection<br />
during a cool, moist growing season.<br />
Next Crop/Prevention<br />
• Do not purchase or accept infected<br />
nursery stock.<br />
BOTRYTIS BLIGHT ................................................................................................................................................................................................................................................................................... 79
COOLEY<br />
SPRUCE GALL<br />
ADELGID ON<br />
SPRUCE<br />
Adelges cooleyi<br />
(Gillette)<br />
Terminal galls of Cooley<br />
spruce gall adelgid. Courtesy<br />
of Cathy Thomas, PDA<br />
Hosts<br />
• Colorado blue spruce<br />
• Occasionally found on other spruces<br />
• Alternate host: Douglas-fi r (see Needle<br />
Discoloration and Injury section <strong>for</strong><br />
Cooley spruce gall adelgid on Douglas-fi r<br />
on page 29)<br />
Damage Potential<br />
• Low<br />
Symptoms and Signs<br />
Throughout the Year<br />
• Green, purple, or brown, pineapple- or<br />
pinecone-shaped galls, 1½–2½ inches<br />
(4–6 cm) long, on the tip of new growth<br />
Causes of Similar Symptoms<br />
• None; galls may be mistaken <strong>for</strong> cones<br />
Identifi cation<br />
The Cooley spruce gall adelgid is a small,<br />
soft-bodied, black insect that may be winged<br />
or wingless. It possesses piercing-sucking<br />
mouthparts to suck sap from its host. On<br />
spruce trees, its presence can be identifi ed<br />
by the pineapple- or pinecone-shaped swelling<br />
or gall. This gall is only found at the tip<br />
of new growth. Galls are green or purple in<br />
color and turn brown as they dry out in the<br />
summer. When dried, they may be confused<br />
with cones.<br />
Figure 1. Overwintering<br />
nymph in bark crevice.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Biology and Life Cycle<br />
The Cooley spruce gall adelgid overwinters<br />
as immature females. On spruce, these overwintering<br />
<strong>for</strong>ms can be found in the bark<br />
crevices of twigs (Fig. 1), just below the buds<br />
or around the base of the buds. In March and<br />
April, the overwintering immatures feed on<br />
sap and gradually swell. They secrete long,<br />
waxy fi laments over their bodies as they<br />
mature (Fig. 2). Shortly be<strong>for</strong>e bud break,<br />
each female produces a cluster of 150–200<br />
eggs inside this mass (Fig. 3). The brownish<br />
nymphs emerge from the eggs as the spruce<br />
buds are opening. They immediately crawl<br />
into the bud and begin feeding on the<br />
elongating needles.<br />
By the time eggs hatch, the bases of<br />
the newly expanding needles have already<br />
started to swell due to feeding by the overwintering<br />
<strong>for</strong>m. When the newly emerged<br />
nymphs feed, they inject a toxin through<br />
their saliva that causes further swelling of the<br />
needle bases. Eventually, the needle bases<br />
fuse together, <strong>for</strong>ming a terminal gall (Fig.<br />
4). Each nymph is contained in an individual<br />
chamber, where they will continue to feed on<br />
the succulent tissue inside the gall (Fig. 5).<br />
The gall turns brown and opens in<br />
midsummer, and the mature nymphs emerge<br />
(Fig. 6). They move to the needles, molt<br />
into winged adults, and will either remain<br />
on the spruce or fl y to a Douglas-fi r tree<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
Spray must occur be<strong>for</strong>e nymphs wax over.<br />
Figure 2. Nymphs covered<br />
with waxy fi laments. Courtesy<br />
of PDA<br />
Figure 3. Cluster of eggs laid<br />
under protective waxy threads.<br />
Courtesy of Whitney Cranshaw,<br />
Colorado State University,<br />
Bugwood.org (#1325023)<br />
SHOOT AND BRANCH INJURY ........................<br />
.............................................................................................................................................................................................................................................................. 80
Figure 4. Fused<br />
needle bases<br />
<strong>for</strong>ming a<br />
terminal gall.<br />
Courtesy of<br />
Cathy Thomas,<br />
PDA<br />
Figure 5.<br />
Adelgids<br />
growing in<br />
chambers<br />
inside a gall.<br />
Courtesy of<br />
Sandy<br />
Gardosik, PDA<br />
(Fig. 7). (See Cooley Adelgid on Douglasfi<br />
r <strong>for</strong> life history of that host.) In fall,<br />
winged adults from Douglas-fi r may travel<br />
back to the spruce tree to produce another,<br />
nondamaging generation. This fall generation,<br />
which includes both male and female<br />
adelgids, will produce the overwintering<br />
<strong>for</strong>ms by mid-October. While the Cooley<br />
spruce gall adelgid alternates its life cycle on<br />
two different hosts, it does not require both<br />
hosts to complete its life cycle. It may cycle<br />
continuously on spruce or Douglas-fi r.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• When planting, separate Colorado blue<br />
spruce and Douglas-fi r trees; this will not<br />
eliminate problems, but it may help lessen<br />
the severity.<br />
Preseason<br />
• Scout <strong>for</strong> brown galls in late fall, winter,<br />
and early spring to note which trees had<br />
a problem.<br />
• Scout <strong>for</strong> overwintering nymphs using<br />
a 15X hand lens.<br />
Growing Season<br />
• Growing degree days: Recommended<br />
control against nymphs should occur<br />
in the spring as nymphs begin to swell,<br />
be<strong>for</strong>e waxing over, at 22–81 GDDs and<br />
then in the fall at 2,800–3,000 GDDs.<br />
• Threshold level: Treatment is warranted<br />
when 5 percent of trees have 10 or more<br />
galls.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• Encourage natural predators such as lacewings,<br />
predatory bugs, and fungi. Refer to<br />
Appendix B: Biological Controls Photo<br />
Chart <strong>for</strong> pictures.<br />
Mechanical<br />
• Pruning: Remove and destroy unopened<br />
green galls on trees be<strong>for</strong>e the release of<br />
mature nymphs (usually mid-July).<br />
Biorational<br />
• Dormant oil: To control overwintering<br />
nymphs, apply in early spring or late fall<br />
(late October/early November) when trees<br />
are not actively growing.<br />
— Only apply oil when temperatures are<br />
above freezing.<br />
— Oil will remove “bloom,” or blue color,<br />
from blue specimens.<br />
Chemical<br />
• Spring insecticide: Apply in mid-April<br />
when greenish-black adelgids are still<br />
exposed, be<strong>for</strong>e waxy fi laments cover the<br />
immature females, and eggs are present;<br />
temperature should be above freezing.<br />
• Fall insecticide: Apply in late September<br />
through October; thoroughly cover<br />
branches and buds. This is the most<br />
reliable time to spray.<br />
• A general rule <strong>for</strong> fall application is to<br />
wait until after the fi rst frost to guarantee<br />
that nymphs will be settled when the<br />
spray is applied.<br />
Next Crop/Prevention<br />
• Only plant pest-free trees from a reputable<br />
source.<br />
Figure 6. Browned galls after<br />
release of adelgids. Courtesy of<br />
Whitney Cranshaw, Colorado<br />
State University, Bugwood.org<br />
(#5369749)<br />
Figure 7. Winged adult adelgid.<br />
Courtesy of Whitney Cranshaw,<br />
Colorado State University,<br />
Bugwood.org (#1326081)<br />
COOLEY SPRUCE GALL ADELGID ON SPRUCE ...................................................................................................................................................................................................................................... 81
DIPLODIA<br />
(SPHAEROPSIS)<br />
TIP BLIGHT OF<br />
PINES AND<br />
OTHER<br />
CONIFERS<br />
Diplodia pinea (Desm.)<br />
J. Kickx f. (<strong>for</strong>merly<br />
Sphaeropsis sapinea)<br />
Shoots turning yellow green<br />
and then brown from<br />
Diplodia. Courtesy of<br />
Tracey Olson, PDA<br />
Hosts<br />
• Austrian, red, Scotch, and other two- or<br />
three-needled pines<br />
• Rarely on Douglas-fi r and spruce<br />
Damage Potential<br />
• Moderate–high<br />
Symptoms and Signs<br />
• Brown, yellow, gray, or straw-colored<br />
needles at tip of current season’s growth;<br />
needles usually stunted and shoot may<br />
curl<br />
• Branch dieback<br />
• Small, black fruiting bodies on needles,<br />
cones, or shoot tissue<br />
• Cankers on stems or branches<br />
• Oozing resin that adheres to blighted<br />
needles<br />
Causes of Similar Symptoms<br />
• Pales weevil feeding<br />
• European pine shoot moth<br />
• Nantucket pine tip moth<br />
• Pine shoot beetle<br />
• Scleroderris canker<br />
• Winter drying<br />
• Drought<br />
Identifi cation<br />
Diplodia (<strong>for</strong>merly Sphaeropsis) tip blight<br />
is caused by the fungus Diplodia pinea. It<br />
is the most common and severe disease of<br />
pines in Pennsylvania and attacks trees of<br />
all ages. Tips of infected current-year shoots<br />
will blight. Needles on these dead tips are<br />
usually tan to straw colored, shorter than<br />
normal, and typically remain attached.<br />
Figure 1. Mature fruiting bodies embedded in<br />
the needles. Courtesy of Joseph O’Brien, USDA<br />
Forest Service, Bugwood.org (#5051004)<br />
When the fungus attacks larger branches or<br />
the main trunk through wounds, misshapen<br />
tops and even branch or tree death can<br />
result. Cankers are generally found only on<br />
mature trees, while tip blight attacks trees of<br />
any age.<br />
<strong>Tree</strong>s are most susceptible to infection<br />
from the time buds begin to open until<br />
the needles are fully elongated. Once the<br />
fungus enters the needle, it kills that tissue<br />
very quickly. Symptoms are visible on the<br />
current season’s growth and on second-year<br />
cones. Infected needles will be stunted, straw<br />
colored, and occasionally glued together with<br />
hardened resin. Cankers may be found on the<br />
fi rst branch whorl of infected twigs. Beginning<br />
in late summer, minute, black, fruiting<br />
bodies (pycnidia) are visible with a 10X<br />
hand lens on needles, cones, and tissue found<br />
attached or unattached to the tree. Woody<br />
tissue beneath cankers is a light brown to<br />
amber color when bark is stripped away. If<br />
the wood beneath these cankers is gray to<br />
black in color, Atropellis may be present.<br />
Biology and Life Cycle<br />
The fungus overwinters in infected needles,<br />
cones, and woody tissue both on and beneath<br />
the tree (Fig. 1). During wet weather<br />
from March through September, the fruiting<br />
bodies mature and release brown, oval<br />
spores (Fig. 2). The spores are distributed<br />
by wind, water, animals, and people to the<br />
new growth, where they germinate on the<br />
needles. The fungus enters needles through<br />
the stomata or may enter branches through<br />
wounds caused by hail, insects, or pruning.<br />
The infection reaches the base of the needle<br />
in a matter of hours, leaving a small, brown<br />
lesion with a resin drop at the point of entry.<br />
Figure 2. Fruiting bodies embedded in bark surface<br />
of twigs. Courtesy of John W. Schwandt,<br />
USDA Forest Service, Bugwood.org (#1241510)<br />
SHOOT AND BRANCH INJURY ........................<br />
.............................................................................................................................................................................................................................................................. 82
The fungus continues to grow into the<br />
twig and results in browning of the attached<br />
needles and subsequent cankers<br />
on the twig (Fig. 3). Needle elongation is<br />
diminished after infection and dying shoots<br />
turn yellow-green be<strong>for</strong>e becoming straw<br />
colored. A girdling canker is produced when<br />
the disease reaches twigs, branches, and the<br />
main trunk. This canker may also exhibit<br />
resin fl ow. Tissue above the canker dies and<br />
major portions of the tree may be killed as<br />
a result.<br />
In the second year, cones can become<br />
infected (Fig. 4). While this does not harm<br />
the tree in any way, infected cones serve as<br />
a large reservoir of spores and contribute<br />
to the spread of the disease. This disease is<br />
present year-round.<br />
Douglas-fi r and spruces have occasionally<br />
been observed with Diplodia tip blight<br />
(Fig. 5). In most cases, this has been a result<br />
of unusual circumstances that have high<br />
disease pressure due to adjacent infected<br />
pines in windrows or nursery blocks.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant disease-free stock.<br />
• Avoid planting susceptible species on sites<br />
where they may be more prone to insect<br />
injury, disease, or stressed conditions.<br />
• Do not plant trees near an area that is<br />
already in infected with Diplodia.<br />
Preseason<br />
• Maintain tree vigor throughout the year<br />
with adequate water and fertilization.<br />
• Mow weeds and area around trees to allow<br />
<strong>for</strong> air circulation. Avoid mower or string<br />
trimmer damage.<br />
Growing Season<br />
• Control insects and other pests to reduce<br />
stress level of trees and potential infection<br />
sites from wounds created by feeding.<br />
• Scouting:<br />
— Late spring/early summer: Randomly<br />
select 50 trees (of any age) and look<br />
<strong>for</strong> stunted, curled, or dead shoots of<br />
current-year growth. Tag or mark the<br />
trees.<br />
— In fall, scout tagged trees <strong>for</strong> fruiting<br />
bodies. Look beneath the fascicle<br />
sheath of needles that are straw colored<br />
or held in place with resin.<br />
— If more than 10 percent of scouted<br />
trees are unfi t <strong>for</strong> sale due to Diplodia,<br />
consider treating the entire plantation<br />
with fungicide next spring.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Prune and remove infected material<br />
(twigs, branches, cones) during dry weather<br />
when fruiting bodies are not releasing<br />
spores. Remove and burn or bury pruned<br />
material.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Apply appropriate fungicide in early<br />
spring when candles begin to elongate.<br />
Continue applications at 1- to 2-week<br />
intervals until needles reach full size<br />
(usually two to four sprays).<br />
• Note: Fungicide application will not protect<br />
seed cones from becoming infected.<br />
No recommendations are available at this<br />
time to prevent infection of seed cones.<br />
Next Crop/Prevention<br />
• Prevent new foliage from becoming<br />
infected by using measures listed above.<br />
Figure 3. Canker causing twig<br />
girdling and leading to tip<br />
dieback. Courtesy of Tracey<br />
Olson, PDA<br />
Figure 4. Infected second-year<br />
cones serving as a source of<br />
infectious spores. Courtesy<br />
of USDA Forest Service North<br />
Central Research Station<br />
Archive, Bugwood.org<br />
(#1406026)<br />
Figure 5. Diplodia infection on<br />
Douglas-fi r. Courtesy of Tracey<br />
Olson, PDA<br />
DIPLODIA (SPHAEROPSIS) TIP BLIGHT OF PINES AND OTHER CONIFERS ........................................................................................................................................................................................... 83
EASTERN<br />
PINE WEEVIL<br />
(FORMERLY<br />
KNOWN AS<br />
NORTHERN<br />
PINE WEEVIL)<br />
Pissodes nemorensis<br />
Germar<br />
Eastern pine weevil adult<br />
feeding damage. Courtesy<br />
of Wood Johnson, USDA<br />
Forest Service, Bugwood.org<br />
(#1113049)<br />
Hosts<br />
• Weakened, dead, or dying trees<br />
• Pine<br />
• Occasionally on spruce and Douglas-fi r<br />
Damage Potential<br />
• Moderate–severe<br />
Symptoms and Signs<br />
• Discoloration and browning (fl agging) of<br />
small branches or twigs<br />
• Small, circular holes in the main trunk or<br />
base of lower branches<br />
• Sap excreting from circular holes<br />
• “Chip cocoons” containing mature larvae<br />
or pupae under the bark on lower half of<br />
the tree; not found below root collar area<br />
Causes of Similar Symptoms<br />
• Pales weevil<br />
• White pine weevil<br />
Identifi cation<br />
Eastern pine weevil adults have long snouts<br />
typical of all weevils. The body is ½– 5 ⁄8 inch<br />
(12–16 mm) long and 1 ⁄8–¼ inch (3–6 mm)<br />
wide. Females are larger than the males.<br />
The main body is covered with scalelike<br />
hairs varying from medium brown to almost<br />
black. The wing coverings have noticeable<br />
patches of white and gold scales on the<br />
rear half. Frequently, these patches are not<br />
joined.<br />
The legless larvae are white with medium<br />
brown heads. They have sparse yellow hairs<br />
and are slightly “C” shaped. A mature larva<br />
is about ½ inch (12 mm) long. Mature<br />
larvae and pupae are found in characteristic<br />
“chip cocoons” under the bark of the tree.<br />
These structures are composed of excelsiorlike<br />
strands of wood removed by the mature<br />
larvae.<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Eastern pine weevil and white pine<br />
weevil are largely inseparable based only on<br />
appearance of the adults, larvae, and pupae.<br />
The two species are often found together in<br />
alcohol-and-turpentine-baited pyramidal, or<br />
Tedder’s, traps used by growers to pinpoint<br />
the emergence time of white pine weevil.<br />
Eastern pine weevil is slightly larger in all<br />
stages, but separation of the two species is<br />
diffi cult even <strong>for</strong> an entomologist. However,<br />
the two species reproduce in different parts<br />
of the tree, which is a reliable tool <strong>for</strong> identifi<br />
cation. White pine weevil will only be<br />
found above the second whorl of branches.<br />
Eastern pine weevil is found on the lower<br />
trunk down to the root collar area as well as<br />
under the bark of stumps.<br />
Biology and Life Cycle<br />
Most eastern pine weevils overwinter as<br />
adults in the soil at the base of trees but<br />
are occasionally found near ground level<br />
in cracks and crevices in the outer bark.<br />
Very few will overwinter as larvae or pupae<br />
in the inner bark of infested trees. Adults<br />
resume activity in April and begin to feed<br />
on stumps, trunks, and large branches of<br />
healthy host trees (Fig. 1). They chew a<br />
small, circular hole in the outer bark and<br />
insert their mouthparts to feed on the inner<br />
bark. Young trees and seedlings are also<br />
attractive hosts in the spring and may be<br />
killed by this feeding.<br />
Figure 1.<br />
Adult<br />
feeding on<br />
a stump.<br />
Courtesy of<br />
Rayanne D.<br />
Lehman, PDA<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
SHOOT AND BRANCH INJURY ........................<br />
.............................................................................................................................................................................................................................................................. 84
After continuously feeding <strong>for</strong> about<br />
3 weeks, the weevils move to appropriate<br />
breeding sites to mate and lay eggs. These<br />
sites include fallen trees, dying or dead trees,<br />
stumps, and sometimes trunks of stressed<br />
transplants. Females deposit eggs singly in<br />
holes chewed in the bark, preferring branch<br />
nodes to open bark areas. Eggs are commonly<br />
deposited on the lower portions of the tree,<br />
often in the shadiest spot. Oviposition is<br />
complete by midsummer, around the time<br />
the adults die. Eggs hatch in about 8 days<br />
and young larvae immediately begin feeding<br />
in the cambial layer (Fig. 2). They will<br />
continue to feed along the grain of the wood,<br />
making galleries in the inner bark. Prior to<br />
pupation, larvae remove wood fi bers from<br />
the xylem to create a chip cocoon in the<br />
outer bark (Fig. 3). If they pupate in small<br />
transplants, the chip cocoons will be in the<br />
inner wood (Fig. 4). Larvae and pupae are<br />
never found in the leader (unlike the white<br />
pine weevil) and never below the soil line<br />
(unlike the pine root collar weevil). Larvae<br />
are commonly found throughout the growing<br />
season.<br />
The majority of adults emerge 2 months<br />
after eggs are laid. Adult emergence starts<br />
in early July and can span several months<br />
due to the long oviposition period and<br />
occasional immature stages that overwinter.<br />
Most eastern pine weevils complete a<br />
generation in 1 year, but a few individuals<br />
will require 2 years. Following emergence,<br />
adults feed briefl y on the bark of available<br />
stumps, healthy trees, and seedlings be<strong>for</strong>e<br />
overwintering (Fig. 5).<br />
Figure 2. Larva feeding under the bark of a<br />
dying tree. Courtesy of PDA<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Delay planting <strong>for</strong> 2 years in an area<br />
where pines were recently harvested.<br />
• Remove and destroy weakened pines in<br />
perimeter of block.<br />
Preseason<br />
• Remove and destroy stumps.<br />
~or~<br />
• Treat stumps from the previous season’s<br />
harvest be<strong>for</strong>e adult weevils become<br />
active in spring.<br />
• Growing degree days: Overwintering<br />
adults become active from 7 to 100 GDDs.<br />
• Destroy cull piles and weakened or dying<br />
trees be<strong>for</strong>e weevils become active in<br />
spring. These weevils are attracted to the<br />
resins in stumps and slash and will fl y up<br />
to several miles to seek breeding material.<br />
Growing Season<br />
• Threshold level: Treat a plantation with<br />
fi ve or more fl agged tips per tree.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Remove dying or dead trees in early spring<br />
since they are good oviposition sites.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• In early spring, treat freshly cut stumps<br />
and the surrounding soil with a registered<br />
insecticide to control ovipositing adults.<br />
• If damage is severe, apply foliar sprays in<br />
late summer to control adults feeding on<br />
mature trees, transplants, and seedlings.<br />
Next Crop/Prevention<br />
• Remove any damaged or highly susceptible<br />
trees surrounding the plantation<br />
be<strong>for</strong>e planting.<br />
Figure 3. Larvae in a chip<br />
cocoon. Courtesy of Gerald J.<br />
Lenhard, Louisiana State<br />
University, Bugwood.org<br />
(#0014079)<br />
Figure 4. Pupa in a chip cocoon.<br />
Courtesy of Gerald J. Lenhard,<br />
Louisiana State University,<br />
Bugwood.org (#0007009)<br />
Figure 5. Adult feeding on a<br />
twig. Courtesy of Rayanne D.<br />
Lehman, PDA<br />
EASTERN PINE WEEVIL ........................................................................................................................................................................................................................................................................... 85
EASTERN<br />
SPRUCE GALL<br />
ADELGID<br />
Adelges abietis<br />
(Linnaeus)<br />
Adelgid nymphs maturing<br />
inside a protective gall.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
Hosts<br />
• Norway spruce (Picea abies)<br />
• White spruce (P. glauca)<br />
Damage Potential<br />
• Moderate<br />
Symptoms and Signs<br />
Be<strong>for</strong>e and at Bud Break<br />
• Stem mothers and small masses of eggs<br />
covered with white, woolly wax<br />
Throughout the Year<br />
• Green- or brown-colored galls at the base<br />
of new shoots; growth beyond gall may be<br />
dead or stunted<br />
Causes of Similar Symptoms<br />
• Cooley spruce gall adelgid (galls will be<br />
found at tips of new growth)<br />
Identifi cation<br />
This insect is diffi cult to fi nd but can easily<br />
be identifi ed by the gall it <strong>for</strong>ms. In spring<br />
as the new growth elongates, galls resembling<br />
small pineapples, ½ –1 inch (1.0–2.5<br />
cm) long, are <strong>for</strong>med at the base of the<br />
new growth—in contrast to Cooley spruce<br />
galls, which <strong>for</strong>m at the end of the new<br />
shoot. New growth will continue beyond<br />
the gall, somewhat obscuring the gall from<br />
view. Initially, galls are green, but they turn<br />
brown and open in late summer, releasing<br />
the nymphs. Both previous and current-year<br />
galls may be visible on trees. The galls may<br />
cause stunting and death of shoots, resulting<br />
in disfi gurement of the branches and tree,<br />
but they rarely kill the tree.<br />
Overwintering immature females can be<br />
found from fall through early spring at the<br />
base of the terminal and lateral buds and in<br />
bark crevices of twigs. They are dark and<br />
may have a faint ring of white, waxy material<br />
surrounding their bodies. As bud break<br />
approaches, they will increase the waxy<br />
material be<strong>for</strong>e depositing eggs. A 15X hand<br />
lens is required to locate overwintering<br />
eastern spruce gall adelgids.<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Biology and Life Cycle<br />
The eastern spruce gall adelgid overwinters<br />
as an immature female called a stem mother<br />
(Fig. 1). As bud break approaches, the stem<br />
mother matures and secretes white, woolly<br />
wax around her body (Fig. 2). She deposits<br />
100–200 greenish-brown eggs in this mass<br />
(Fig. 3).<br />
Figure 1. Overwintering immature<br />
females (stem mothers). Courtesy of<br />
Rayanne D. Lehman, PDA<br />
Figure 2. Female secreting waxy<br />
fi laments to cover her body. Courtesy<br />
of PDA<br />
Figure 3. Eggs under white, woolly<br />
wax made by the stem mother.<br />
Courtesy of Sandy Gardosik, PDA<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
SHOOT AND BRANCH INJURY ........................<br />
.............................................................................................................................................................................................................................................................. 86
At bud break the eggs hatch into<br />
yellowish-colored nymphs, which move to<br />
the new growth and begin feeding. This<br />
feeding causes abnormal twig growth, which<br />
results in gall <strong>for</strong>mation (Figs. 4 and 5).<br />
Figure 4. Purplish growth indicating the start<br />
of a gall on new growth. Courtesy of Tracey<br />
Olson, PDA<br />
Figure 5. Gall of eastern spruce gall adelgid.<br />
Courtesy of Minnesota Department of<br />
Natural Resources Archive, Bugwood.org<br />
(#4212052)<br />
The adelgids remain safe in the gall<br />
until August/September when the gall turns<br />
brown and opens to release mature nymphs<br />
(Figs. 6 and 7). These nymphs mature to<br />
winged, egg-laying females; there are no<br />
males. These females generally remain on<br />
the same tree, depositing their eggs near the<br />
tips of needles. These second-generation<br />
eggs result in immature females, which will<br />
overwinter near buds (Fig. 8).<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant tree varieties that are resistant to or<br />
are not hosts of eastern spruce gall adelgid.<br />
• Remove and destroy heavily infested trees<br />
within and surrounding the plantation.<br />
Preseason<br />
• Scout <strong>for</strong> brown galls on trees from the<br />
previous year’s infestation; monitor these<br />
trees closely <strong>for</strong> exposed stem mothers<br />
(be<strong>for</strong>e covered with waxy fi laments).<br />
• Be<strong>for</strong>e buds double in size and bud scales<br />
loosen, apply a dormant oil or spring<br />
insecticide to manage overwintering<br />
females.<br />
— Only apply oil when temperatures are<br />
above freezing.<br />
— Oil will remove “bloom,” or blue color,<br />
from blue specimens.<br />
• Around bud break time, scout <strong>for</strong> white,<br />
woolly egg masses near the buds.<br />
Growing Season<br />
• Threshold level: At this time, no determined<br />
threshold number exists <strong>for</strong> the<br />
eastern spruce gall adelgid.<br />
• Growing degree days: Recommended<br />
control against nymphs should occur in<br />
spring, be<strong>for</strong>e nymphs wax over, at 22–170<br />
GDDs and in fall at 2,800–3,000 GDDs<br />
• Scout <strong>for</strong> green galls; remove and destroy<br />
them whenever possible be<strong>for</strong>e they turn<br />
brown and open in late summer.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Remove green galls be<strong>for</strong>e they open in<br />
late summer.<br />
Biorational<br />
• Apply dormant oil in spring to manage<br />
overwintering females (only when<br />
temperatures are above freezing).<br />
Chemical<br />
• Apply a fall insecticide or dormant oil<br />
from mid-September to October to<br />
manage overwintering stages.<br />
Next Crop/Prevention<br />
• Only plant pest-free trees from a reputable<br />
source.<br />
Figure 6. Browned gall after<br />
release of adelgids. Courtesy<br />
of E. Brad<strong>for</strong>d Walker, Vermont<br />
Department of Forests, Parks,<br />
and Recreation, Bugwood.org<br />
(#0907022)<br />
Figure 7. Gall damage on<br />
Norway spruce. Courtesy of<br />
Pennsylvania DCNR Forestry<br />
Archive, Bugwood.org<br />
(#5018059)<br />
Figure 8. Overwintering<br />
females around buds. Courtesy<br />
of Rayanne D. Lehman, PDA<br />
EASTERN SPRUCE GALL ADELGID ......................................................................................................................................................................................................................................................... 87
GALL RUSTS<br />
Eastern<br />
(Pine-Oak Gall Rust),<br />
Cronartium<br />
quercuum (Berk.)<br />
Miyabe ex Shirai<br />
Western<br />
(Pine-Pine Gall Rust),<br />
Endocronartium<br />
harknessii<br />
(J. P. Moore)<br />
Y. Hiratsuka<br />
Galls may produce spores<br />
each spring <strong>for</strong> up to<br />
10 years. Courtesy of<br />
Tracey Olson, PDA<br />
Hosts<br />
• Two- and three-needle pines: Scotch,<br />
Austrian, Jack, Mugo, red, and Virginia<br />
• Alternate hosts: oaks <strong>for</strong> eastern gall rust<br />
Damage Potential<br />
• Moderate–high<br />
Symptoms and Signs<br />
• Stunting, de<strong>for</strong>mation, and dieback of<br />
twigs and branches<br />
• Woody, globelike galls on branches or<br />
stems<br />
• Yellow-orange aecia (fruiting bodies)<br />
on surface of mature galls in spring<br />
Causes of Similar Symptoms<br />
• None<br />
Identifi cation<br />
Western (pine-pine) gall rust is the more<br />
common of the two in Pennsylvania, but<br />
eastern (pine-oak) gall rust can also occur.<br />
Both produce visible, globe-shaped galls<br />
on the stems or branches. The galls will<br />
increase in size each year and can end up<br />
being several inches in diameter. Around<br />
mid-May on 2-year-old and older branches,<br />
the blisterlike membrane of the fruiting<br />
structures (aecia) ruptures, covering the<br />
surface with a mass of powdery, yelloworange<br />
spores (aeciospores). These spores<br />
are dispersed in the wind and will infect<br />
new plants. Western gall rust can easily<br />
re-infect the same tree and may result in<br />
multiple galls on the same tree and even<br />
the same branch. If a tree has a signifi cant<br />
amount of galls, stunting and dieback of<br />
branches may occur and lead to death of<br />
the tree. Western gall rust may be especially<br />
lethal to seedlings.<br />
Biology and Life Cycle<br />
Eastern (pine-oak) gall rust requires two<br />
hosts: pine and oak. The spores released in<br />
the spring from the galls on pines can only<br />
infect the leaves of oak, the alternate host.<br />
<strong>Tree</strong>s in the red and black oak group are<br />
especially susceptible. Once young leaves<br />
of oaks have become infected, a repeating<br />
spore stage occurs and the disease intensifi<br />
es. By midsummer, spores that are capable<br />
of infecting only pines are then produced on<br />
the oak leaves. Absence of oaks in the growing<br />
area will break the required two-host<br />
life cycle and minimize the threat of this<br />
disease. Western (pine-pine) gall rust does<br />
not require an alternate host and is spread<br />
directly from one pine to another, infecting<br />
only new growth during the spring sporulation<br />
period.<br />
For both diseases, spores are wind dispersed<br />
and infect new needles. The fungus<br />
grows into the shoots, where it establishes<br />
in the woody tissue of branches (Fig. 1) and<br />
possibly the main trunk (Fig. 2). Small galls<br />
may be visible after the fi rst year; however,<br />
it may take up to 3 years <strong>for</strong> them to become<br />
large enough to be noticed (Fig. 3).<br />
Figure 1. Western gall rust on a branch (not<br />
sporulating). Courtesy of Susan K. Hagle, USDA<br />
Forest Service, Bugwood.org (#1241721)<br />
Figure 2. Gall rust on the main trunk. Courtesy<br />
of Joseph O’Brien, USDA Forest Service,<br />
Bugwood.org (#5034007)<br />
Figure 3. Gall <strong>for</strong>ming at an infection site.<br />
Courtesy of Tracey Olson, PDA<br />
SHOOT AND BRANCH INJURY ........................<br />
.............................................................................................................................................................................................................................................................. 88
Galls are <strong>for</strong>med due to an increase in<br />
wood production at the infection site. After<br />
the second year of infection, yellow-orange<br />
fruiting structures (aecia) will <strong>for</strong>m on<br />
the surface of the gall (Fig. 4). Galls may<br />
actively produce spores each spring <strong>for</strong> up<br />
to 10 years.<br />
Figure 4. Powdery, yellow-orange spores on the<br />
surface of a gall. Courtesy of Tracey Olson, PDA<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Avoid planting in fi elds adjacent to <strong>for</strong>est<br />
stands of oak or pines that show evidence<br />
of infection.<br />
• Plant disease-free nursery stock from a<br />
reputable source.<br />
Preseason<br />
• Inspect seedling beds one year after<br />
planting. Remove any seedlings showing<br />
signs of galls.<br />
• In older plantings, remove branches<br />
with galls prior to active sporulation in<br />
mid-May.<br />
• If a tree is heavily infected with multiple<br />
galls or trunk dieback is visible, completely<br />
remove and destroy the tree be<strong>for</strong>e<br />
sporulation.<br />
Growing Season<br />
• Scout fi elds to look <strong>for</strong> trees with branch<br />
and stem galls. For those that cannot be<br />
removed, monitor <strong>for</strong> presences of fruiting<br />
bodies.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Remove seedlings exhibiting galls from<br />
the fi eld or cut out branches with galls<br />
from older, established trees. Do not move<br />
during period of spore production.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• For western (pine-pine) gall rust, apply<br />
a registered fungicide to new growth of<br />
pines at the time of bud break to prevent<br />
infection. This must be applied be<strong>for</strong>e the<br />
fungus sporulates and releases the orange<br />
spores.<br />
Next Crop/Prevention<br />
• Buy disease-free and/or resistant stock<br />
from a reputable nursery.<br />
• Rotate crop in the planting block to a<br />
nonsusceptible tree variety.<br />
GALL RUSTS ............................................................................................................................................................................................................................................................................................ 89
PALES WEEVIL<br />
Hylobius pales (Herbst)<br />
Flagged branches are a<br />
symptom of pales weevil<br />
feeding. Courtesy of Sandy<br />
Gardosik, PDA<br />
Hosts<br />
• Pines preferred, Scotch more so than<br />
eastern white<br />
• Occasionally on Douglas-fi r, fi r, spruce,<br />
and other conifer species<br />
Damage Potential<br />
• Moderate–high<br />
Symptoms and Signs<br />
• Dead seedlings with missing bark near<br />
soil line<br />
• Small holes or pits in the bark from<br />
feeding that may fi ll with resin if<br />
infestation is light<br />
• Yellow needles at branch tips that turn<br />
reddish brown, giving the tree a “fl agged”<br />
appearance; bark will be missing behind<br />
the dead tissue<br />
• White, C-shaped larvae under the bark of<br />
stumps and roots<br />
• Adult weevils in the leaf litter surrounding<br />
trees or on stumps in early spring<br />
Causes of Similar Symptoms<br />
• Eastern pine shoot borer<br />
• Diplodia (Sphaeropsis) shoot blight<br />
• Northern pine weevil<br />
• White pine blister rust<br />
• Pine shoot beetle<br />
• Wood borers<br />
• Bark beetles<br />
Identifi cation<br />
The adult pales weevil is a large, robust<br />
insect measuring ¼– 2 ⁄5 inch (6–10 mm)<br />
long with a prominent snout. The weevil is<br />
black to dark reddish brown in color with<br />
very small patches of yellow-white scales<br />
on its head and wing coverings. In early<br />
spring, adults are frequently found on and<br />
around 1- to 2-year-old stumps. The larvae<br />
are cream colored, legless, and “C” shaped.<br />
They have a light brown head capsule and<br />
are found burrowed into the cambium tissue<br />
of stumps and roots.<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Adult pales weevils are particularly<br />
damaging to eastern white pine, Douglas-fi r,<br />
and true fi rs. The weevils feed on terminal<br />
shoots, consuming the bark down to the<br />
wood and girdling and killing the shoot.<br />
This fl agging can seriously disfi gure marketable<br />
trees (Fig. 1). Larval feeding is generally<br />
restricted to 1- to 2-year-old stumps and dying<br />
trees of Scotch, Austrian, and red pine.<br />
Larvae and pupae may be found on larger<br />
roots below the soil surface. Larvae pupate in<br />
chambers plugged with wood fi bers.<br />
Figure 1.<br />
Evidence of<br />
weevil feeding<br />
indicated<br />
by fl agging.<br />
Courtesy of<br />
Rayanne D.<br />
Lehman, PDA<br />
Biology and Life Cycle<br />
Pales weevils overwinter as adults in the soil<br />
and become active between April and June<br />
(Fig. 2). They feed <strong>for</strong> several weeks on seedlings<br />
and the tender bark on twigs of more<br />
mature trees (Figs. 3 and 4). Feeding occurs<br />
at night, while the weevils take refuge in the<br />
leaf litter and under logs during the day.<br />
Figure 2. Adult pales weevil feeding on<br />
bark of a twig after spring emergence.<br />
Courtesy of PDA<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
SHOOT AND BRANCH INJURY ........................<br />
.............................................................................................................................................................................................................................................................. 90
After feeding, the weevils mate and<br />
females lay pearly white eggs in the subterranean<br />
roots of pine stumps or dying trees.<br />
Eggs hatch within 10–14 days and the larvae<br />
feed beneath the bark until they mature<br />
in early fall (Fig. 5).The larvae pupate in<br />
a chip cocoon (Fig. 6) be<strong>for</strong>e emerging as<br />
adults in August–October. These adults feed<br />
<strong>for</strong> 2–3 weeks be<strong>for</strong>e seeking overwintering<br />
sites in the soil around fresh-cut stumps or<br />
beneath dying trees. One generation occurs<br />
per year.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Delay planting <strong>for</strong> 2 years in an area<br />
where pines were recently harvested.<br />
Preseason<br />
• Remove and destroy stumps.<br />
~or~<br />
Figure 3. Weevil<br />
feeding damage<br />
on eastern<br />
white pine<br />
branches.<br />
Courtesy of<br />
Brian Schildt,<br />
PDA<br />
Figure 4. Weevil<br />
feeding damage<br />
on Fraser fi r<br />
leader.<br />
Courtesy of<br />
Rayanne D.<br />
Lehman, PDA<br />
• Treat stumps from the previous season’s<br />
harvest be<strong>for</strong>e adult weevils become<br />
active in spring.<br />
• Destroy culled tree piles be<strong>for</strong>e pales<br />
weevils become active in spring. These<br />
weevils are attracted to the resins and will<br />
fl y up to several miles to seek breeding<br />
material. Traps deployed to monitor <strong>for</strong><br />
white pine weevil will also attract pales<br />
weevil adults.<br />
• Growing degree days: Treat stumps<br />
with insecticide to prevent egg laying<br />
at 7–121 GDDs.<br />
Growing Season<br />
• Scout <strong>for</strong> the presence of different life<br />
stages of the weevil.<br />
— For adults: Lay a sheet out under a tree<br />
after dark and shake the tree. Look <strong>for</strong><br />
weevils that fall from the tree to the<br />
sheet.<br />
— For larvae and pupae: Use a knife to<br />
cut back the bark on stumps or the<br />
base of dead trees to look <strong>for</strong> larvae<br />
burrowing through the tissue or pupae<br />
in chip cocoons.<br />
• Scout 50 random trees of any age <strong>for</strong><br />
seedling injury and branch fl agging.<br />
— If seedlings show any weevil injury<br />
or older trees average more than fi ve<br />
fl agged branches, consider treating the<br />
entire plantation.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Remove and burn or chip stumps and<br />
debris.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Dip new seedlings (aboveground portion)<br />
in appropriate insecticide be<strong>for</strong>e planting.<br />
• In early spring, apply an approved pesticide<br />
to stumps from previous cutting<br />
season. Stumps older than 2 years do not<br />
need to be treated.<br />
• If a population is extremely high, apply<br />
an appropriate insecticide to mature trees<br />
in spring between April and May and<br />
again in August and September to kill<br />
bark-feeding weevils.<br />
Next Crop/Prevention<br />
• Remove cut stumps or treat stumps with<br />
an insecticide be<strong>for</strong>e replanting a fi eld.<br />
Figure 5. Larvae feeding under<br />
the bark of a stump. Courtesy<br />
of Rayanne D. Lehman, PDA<br />
Figure 6. Chip cocoon created<br />
by larvae be<strong>for</strong>e pupating into<br />
adults. Courtesy of PDA<br />
PALES WEEVIL ......................................................................................................................................................................................................................................................................................... 91
PINE BARK<br />
ADELGID<br />
Pineus strobi (Hartig)<br />
<strong>Tree</strong> with pine bark adelgid<br />
infestation on the branches<br />
and trunk. Courtesy of Brian<br />
Schildt, PDA<br />
Hosts<br />
• Eastern white pine<br />
• Sometimes on Austrian and Scotch pine<br />
Damage Potential<br />
• Low–moderate (pest seems to have decreased<br />
since appearance of multicolored<br />
Asian lady beetle)<br />
Symptoms and Signs<br />
• Small clumps of white, woolly wax on<br />
candles, branches, or trunks of discolored,<br />
stunted, weakened, or dying trees<br />
• Yellow- or purple-colored insects under<br />
woolly wax<br />
• Dark blue to green, wool-covered nymphs<br />
on growing branches (in May–June)<br />
Causes of Similar Symptoms<br />
• Balsam woolly adelgid<br />
Identifi cation<br />
A tree heavily infested with pine bark<br />
adelgid may have the look of snow on its<br />
branches and trunk (Fig. 1). However, in<br />
<strong>Christmas</strong> tree production, infestations<br />
seldom reach this stage. Instead, buds, elongating<br />
candles, and needle bases will have<br />
the white, woolly mass if pine bark adelgid<br />
is present. This mass may contain a nymph,<br />
a wingless, purplish adult female (about 1 ⁄32<br />
inch long), or up to 25 light yellow-brown<br />
eggs. Winged <strong>for</strong>ms may be present during<br />
the growing season.<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Figure 1. <strong>Tree</strong><br />
with severe<br />
adelgid<br />
infestation.<br />
Courtesy of<br />
USDA<br />
Forest Service<br />
Northeastern<br />
Area Archive,<br />
Bugwood.org<br />
(#1396093)<br />
Biology and Life Cycle<br />
Pine bark adelgids overwinter predominately<br />
as immature females (Fig. 2). By early<br />
spring when temperatures reach 50°F, the<br />
female is mature and begins to produce a<br />
coating of woolly wax (Fig. 3). She deposits<br />
about 25 eggs under the woolly mass be<strong>for</strong>e<br />
dying. When the crawlers emerge from<br />
the eggs, they move to a new location and<br />
begin to feed. Once they have inserted their<br />
mouthparts into the bark, they are no longer<br />
capable of movement.<br />
Both winged and wingless adults result<br />
from the next several generations. Winged<br />
<strong>for</strong>ms will fl y to adjacent trees, while wingless<br />
<strong>for</strong>ms remain on the same trees. Five<br />
generations are thought to occur each year,<br />
but all stages may be present at any given<br />
time during the growing season.<br />
Figure 2.<br />
Nymph on elongating<br />
candle.<br />
Courtesy of<br />
Rayanne D.<br />
Lehman, PDA<br />
Figure 3. Protective waxy, woolly covering<br />
produced by mature females. Courtesy of<br />
Lacy L. Hyche, Auburn University, Bugwood.org<br />
(#1540233)<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
SHOOT AND BRANCH INJURY ........................<br />
.............................................................................................................................................................................................................................................................. 92
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Do not plant eastern white pine, particularly<br />
if mature Austrian and Scotch pine<br />
are growing nearby.<br />
Preseason<br />
• Scout <strong>for</strong> overwintering blue-green<br />
nymphs under white, woolly wax.<br />
Growing Season<br />
• Scout <strong>for</strong> this pest during the entire<br />
season on susceptible trees of all ages.<br />
• Growing degree days: Dormant control of<br />
overwintering immatures should occur at<br />
22–58 GDDs.<br />
• Threshold level: No threshold has been<br />
established. Treat infested trees if adelgids<br />
are found on numerous shoots or coating<br />
the bark and predators are not present.<br />
Many benefi cial insects are attracted to<br />
pine bark adelgid, so use discretion when<br />
applying insecticides <strong>for</strong> control of this<br />
pest. If possible, spot treat infested trees<br />
using a backpack sprayer.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• Encourage benefi cial predators such as<br />
dusty wings, hoverfl ies, and lady beetles<br />
(Fig. 4). This pest appears to have decreased<br />
in occurrence and severity since<br />
the introduction of multicolored Asian<br />
lady beetle (Harmonia axyridis).<br />
Mechanical<br />
• No recommendations are available<br />
at this time.<br />
Biorational<br />
• If benefi cial predators are present, use<br />
insecticidal soap or horticultural oil.<br />
• Dormant oils: Applications made during<br />
the fall and early spring that completely<br />
cover the trunk and branches will kill<br />
overwintering nymphs.<br />
— Only apply oil when temperatures are<br />
above freezing.<br />
— Oil will remove “bloom,” or blue color,<br />
from blue specimens.<br />
Chemical<br />
• Insecticide: Apply a registered product<br />
from mid-April to May when adelgids are<br />
active.<br />
Next Crop/Prevention<br />
• Only plant pest-free trees from a reputable<br />
source.<br />
Figure 4. Seven-spotted lady<br />
beetle feeding on pine bark<br />
adelgid. Courtesy of Sandy<br />
Gardosik, PDA<br />
PINE BARK ADELGID .............................................................................................................................................................................................................................................................................. 93
PINE SHOOT<br />
BEETLE<br />
(OR COMMON<br />
PINE SHOOT<br />
BEETLE)<br />
Tomicus piniperda<br />
(Linnaeus)<br />
Flagging damage from pine<br />
shoot beetle. Courtesy of<br />
Rayanne D. Lehman, PDA<br />
This is a federally regulated pest. A Compliance<br />
Agreement is available to enable<br />
growers within the quarantine area to ship<br />
trees to other states. Consult your state or<br />
federal agency <strong>for</strong> in<strong>for</strong>mation on the pine<br />
shoot beetle quarantine area and shipping<br />
regulations.<br />
Hosts<br />
• Pines, especially Austrian and Scotch<br />
• Occasionally on eastern white pine<br />
Damage Potential<br />
• Moderate–high<br />
Symptoms and Signs<br />
Spring<br />
• Boring dust (reddish in color) on cut trees<br />
or stumps<br />
• Egg and larval galleries beneath the bark<br />
of cut trees or stumps<br />
June Through December<br />
• Shoots on live trees turn yellow or red<br />
• Drooping shoots and round holes<br />
surrounded by pitch<br />
• Shoot completely hollow<br />
Causes of Similar Symptoms<br />
• Other bark beetles<br />
• Diplodia (Sphaeropsis) shoot blight<br />
• Eastern pine shoot borer<br />
• European pine shoot moth<br />
Identifi cation<br />
Pine shoot beetle eggs are approximately 1 ⁄ 25<br />
inch (1 mm) long, oval, smooth, and shiny<br />
white. Larvae are legless, white grubs, up<br />
to 1 ⁄ 5 inch (5 mm) long, with brown heads.<br />
Both eggs and larvae are found inside galleries<br />
under the bark of weakened trees or<br />
stumps. Reddish sawdust may be apparent as<br />
the larvae bore in the inner bark.<br />
Adult beetles are cylindrical, dark brown<br />
or black, and generally shiny. They can<br />
be up to 1 ⁄ 5 inch (5 mm) in length. Newly<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
emerged adults are easiest to fi nd in summer<br />
as they go through their maturation feeding<br />
stage. These new adults will feed inside<br />
current-year shoots until fall when they seek<br />
overwintering sites. They generally feed<br />
within 6 inches (15 cm) or less of the shoot<br />
tip, completely hollowing out the shoot.<br />
This gallery is completely free of sawdust<br />
and frass, in contrast to the gallery of moth<br />
larvae. The entrance/exit hole used by the<br />
adults is round and frequently ringed with<br />
sap and sawdust. Shoots bored by pine shoot<br />
beetles turn yellow and may droop or break<br />
from the tree (Figs. 1 and 2).<br />
Figure 1. Exit hole from summer<br />
maturation feeding site. Courtesy of<br />
Rayanne D. Lehman, PDA<br />
Figure 2. Symptom of pine shoot<br />
beetle infestation. Courtesy of<br />
Rayanne D. Lehman, PDA<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
SHOOT AND BRANCH INJURY ........................<br />
.............................................................................................................................................................................................................................................................. 94
Biology and Life Cycle<br />
Although they can survive as mature larvae<br />
or pupae, pine shoot beetles most often<br />
overwinter as adults in the bark or lower<br />
stems at the base of trees (Fig. 3). In early<br />
spring, adults become active and fl y to<br />
recently cut trees and stumps, attracted to<br />
chemicals released by the trees. Both sexes<br />
create a club-shaped chamber called a gallery<br />
between the bark and sapwood (Fig.<br />
4). The female beetle carves out the gallery,<br />
which runs parallel with the grain of the<br />
wood, while the male ejects the debris from<br />
the tunnel and blocks the opening so other<br />
males cannot enter. Eggs are deposited in<br />
niches created along the wall of the gallery<br />
and hatch 2–3 weeks later. Larvae feed <strong>for</strong><br />
6–8 weeks as they bore horizontally into<br />
cambial tissues be<strong>for</strong>e pupating.<br />
Figure 3.<br />
Adult pine<br />
shoot beetle.<br />
Courtesy of<br />
Jim Stimmel,<br />
PDA,<br />
Bugwood.org<br />
(#2117021)<br />
Figure 4. Adult beetles in a breeding<br />
gallery. Courtesy of Louis-Michel Nageleisen,<br />
Département de la Santé des Forêts, Bugwood<br />
.org (#2101014)<br />
In early summer, new adults emerge<br />
from the breeding site and begin feeding<br />
on current or one-year-old growth of living<br />
trees. This “maturation feeding” can occur<br />
from May through October (Figs. 5 and 6).<br />
Beetles may feed on as many as six shoots<br />
be<strong>for</strong>e overwintering. One generation<br />
occurs per year.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant tree species other than pine.<br />
Preseason<br />
• Chip or burn any culled pines to remove<br />
breeding sites.<br />
• Cut pine stumps low to the ground and<br />
apply appropriate insecticide to prevent<br />
larval development in late April/early<br />
May.<br />
Growing Season<br />
• Scout <strong>for</strong> dead or bent shoots on the<br />
upper half of the tree; shoots may be<br />
yellow or red and will have small, round<br />
holes where beetles entered and exited.<br />
• Clip off and open suspected shoots to<br />
examine <strong>for</strong> pine shoot beetle.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Figure 5. Adult beetle exiting<br />
from a maturation feeding<br />
site. Courtesy of Rayanne D.<br />
Lehman, PDA<br />
Figure 6. Adult beetle inside<br />
a feeding gallery within a<br />
shoot. Courtesy of Rayanne D.<br />
Lehman, PDA<br />
PINE SHOOT BEETLE ............................................................................................................................................................................................................................................................................... 95
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Sanitation is critical to controlling<br />
this pest.<br />
• Cut stumps less than 4 inches (10 cm)<br />
from the ground when harvesting trees or<br />
in the spring be<strong>for</strong>e the new generation<br />
completes development.<br />
• Chip or burn any unused or discarded<br />
trees or branches to minimize breeding<br />
site material.<br />
• Use trap logs to attract breeding parent<br />
beetles by systematically placing freshly<br />
cut pine trees or logs along the edges of<br />
the fi eld in early spring. The trap logs<br />
must be chipped or burned after breeding<br />
occurs but be<strong>for</strong>e new adults emerge.<br />
Specifi c instructions and timetable are<br />
provided with the federal compliance program<br />
guidelines. See the USDA-APHIS/<br />
PPQ Web site on pine shoot beetle (www<br />
.aphis.usda.gov/plant_health/plant_pest_<br />
info/psb/index.shtml) <strong>for</strong> further details<br />
on regulation of this pest.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Apply appropriate insecticide to stumps<br />
to prevent larval development (late April/<br />
early May).<br />
• Apply appropriate insecticide to foliage<br />
timed with the emergence of the new<br />
generation of beetles to help manage<br />
the beetle population at 450–550 GDDs<br />
(usually early–mid-June).<br />
Next Crop/Prevention<br />
• Only plant pest-free trees from a reputable<br />
source.<br />
SHOOT AND BRANCH INJURY .............................................................................................................................................................................................................................................................. 96
Hosts<br />
• Two- and three-needled pines, especially<br />
Scotch<br />
Damage Potential<br />
• Moderate<br />
Symptoms and Signs<br />
• Black sooty mold on surface of bark and<br />
needles; tree may appear darker from<br />
distance<br />
• Clusters of brown and white, helmetshaped<br />
scales on branches<br />
• Presence of stinging insects in and<br />
on trees<br />
• Reduced plant vigor<br />
Causes of Similar Symptoms<br />
• Pine tortoise scale<br />
• Cinara aphids<br />
Identifi cation<br />
Note: Many previous reports of pine tortoise<br />
scale (Toumeyella parvicornis Cockerell) in<br />
Pennsylvania actually refer to this closely<br />
related species. Striped pine scale is the more<br />
common of the two species in Pennsylvania.<br />
The striped pine scale is a soft scale<br />
found on the new growth of pines. The<br />
coverings of adult female scales resemble<br />
miniature helmets. They are reddish brown<br />
and may appear to have one or more cream<br />
or white stripes centrally. This coloration<br />
varies with age and condition of the scales.<br />
The coverings of mature females are ¼ inch<br />
(6.3 mm) long. They are generally found<br />
in clusters on the outer twigs of host trees.<br />
Nymphs or crawlers of this scale are tan<br />
to orange and will be found on the twigs<br />
and needles of new growth. As this insect<br />
feeds on the bark of pines, it excretes a<br />
sugary liquid known as honeydew (Fig. 1).<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Black sooty mold will grow on the bark and<br />
needles coated with the honeydew, giving<br />
the tree a darkened appearance (Fig. 2).<br />
Frequently, ants, bees, and wasps will be<br />
found feeding on this honeydew. A heavy<br />
buildup of sooty mold results in stunting and<br />
chlorotic growth.<br />
Figure 1. Striped pine scale excreting a<br />
drop of honeydew. Courtesy of Brian<br />
Schildt, PDA<br />
Figure 2. Black sooty mold development<br />
resulting from honeydew excretion.<br />
Courtesy of R. Scott Cameron, Advanced<br />
Forest Protection, Inc., Bugwood.org<br />
(#3226096)<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
STRIPED<br />
PINE SCALE<br />
Toumeyella pini (King)<br />
Severe scale crawler<br />
infestation on Scotch pine,<br />
along with a heavy amount<br />
of sooty mold (black coating<br />
on stem and needles).<br />
Courtesy of Brian Schildt,<br />
PDA<br />
STRIPED PINE SCALE .............................................................................................................................................................................................................................................................................. ..................................................................... 71 97
Figure 3. Female striped pine<br />
scales. Courtesy of Brian<br />
Schildt, PDA<br />
Figure 4. Scale crawlers moving<br />
over the top of a female scale.<br />
Courtesy of Rayanne D. Lehman,<br />
PDA<br />
Biology and Life Cycle<br />
Striped pine scale has a single generation<br />
each year in Pennsylvania. Fertilized, immature<br />
females can be found on twigs of<br />
the most recent season’s growth (Fig. 3).<br />
Occasionally, a few mature females may also<br />
be seen overwintering <strong>for</strong> a second season<br />
on last year’s growth. In early spring, the<br />
overwintering nymphs resume feeding and<br />
mature. Females deposit eggs under their<br />
helmet- or tortoise-shaped covering in early<br />
summer. These eggs, which may number<br />
in the hundreds, will hatch over a period<br />
of several weeks in late June or early July.<br />
The newly hatched nymphs, or crawlers, are<br />
very active (Fig. 4). They quickly move to<br />
the new expanding growth and can even be<br />
found on the ground under trees. Crawlers<br />
can attach to insects, birds, animals, people,<br />
and machinery and be easily moved to uninfested<br />
trees. Within a day or two, the newly<br />
emerged crawlers molt, losing their legs and<br />
gaining long mouthparts. Once they settle<br />
to feed, this pest is no longer capable of<br />
moving about on the host. Female scales remain<br />
on the bark of twigs, while male scales<br />
settle on the needles. The males are much<br />
smaller and the winged adults are generally<br />
unnoticed.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant trees with adequate spacing between<br />
them to prevent crawlers from moving<br />
tree to tree on overlapping branches.<br />
• Inspect seedling stock be<strong>for</strong>e planting <strong>for</strong><br />
presence of scales on the young growth.<br />
Preseason<br />
• Scout fi elds of susceptible trees <strong>for</strong> presence<br />
of scales on last season’s growth. Pay<br />
particular attention to trees attractive to<br />
ants and stinging insects.<br />
• If only a few trees show severe infestation,<br />
remove these trees from the fi eld be<strong>for</strong>e<br />
bud break and burn or chip them.<br />
Growing Season<br />
• Monitor known scale infestation <strong>for</strong><br />
presence of emerging scale nymphs.<br />
• Growing degree days: Crawlers emerge at<br />
400–500 GDDs.<br />
• Avoid mowing during crawler emergence<br />
to prevent mechanical spread.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• Encourage naturally occurring parasitoids<br />
and predators. Lacewing larvae and lady<br />
beetles feed on crawlers.<br />
• Do not use a broad-spectrum insecticide<br />
that will kill benefi cial insects.<br />
Mechanical<br />
• Remove and burn or destroy severely<br />
infested trees be<strong>for</strong>e bud break.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Apply dormant oil in fall or in spring<br />
be<strong>for</strong>e bud break.<br />
— Only apply oil when temperatures are<br />
above freezing.<br />
— Oil will remove “bloom,” or blue color,<br />
from blue specimens.<br />
• A single insecticide treatment applied at<br />
crawler emergence in early summer is generally<br />
effective. After fi rst spray, inspect<br />
foliage with a hand lens to determine if<br />
the spray was effective or if a second spray<br />
is necessary.<br />
Next Crop/Prevention<br />
• Purchase and plant scale-free nursery<br />
stock from a reputable company.<br />
SHOOT AND BRANCH INJURY .............................................................................................................................................................................................................................................................. 98
Hosts<br />
• Eastern white pine (Pinus strobus)<br />
• Other fi ve-needled pines<br />
Alternate Hosts<br />
• Currants<br />
• Gooseberry<br />
• Other Ribes species<br />
Damage Potential<br />
• Moderate–high<br />
Symptoms and Signs<br />
On Pine<br />
Year-round:<br />
• Chlorotic needles, stunted growth<br />
• Chlorotic, dead, or dying tree tops or<br />
branches<br />
• Yellow-bordered cankers on trunk or<br />
3- to 4-year-old branches; oozing resin or<br />
rodent feeding may be apparent<br />
• Resin fl ow on main trunk that hardens to<br />
white, orange, or brown<br />
Early spring:<br />
• Orange-yellow blisters breaking through<br />
cankered bark to release spores<br />
Late spring to early summer:<br />
• Sticky, yellow fl uid produced from<br />
yellow-brown blister on canker; fl uid<br />
will blacken after a short time<br />
Fall:<br />
• Patches of yellow or brown bark on<br />
young growth; area may be swollen but<br />
progresses to spindle-shaped canker by<br />
second year of infection<br />
On Ribes Species<br />
Summer:<br />
• Orange spores on underside of leaf; upper<br />
surface may have yellow, diffused spots<br />
Late summer to early fall:<br />
• Brown, hairlike projections on underside<br />
of leaf<br />
Figure 1. Flagging as a result of cankers girdling<br />
a branch. Courtesy of John W. Schwandt, USDA<br />
Forest Service, Bugwood.org (#1241718)<br />
Causes of Similar Symptoms<br />
• Fungal root rots<br />
• Pales weevil<br />
• Pine root collar weevil<br />
Identifi cation<br />
White pine blister rust is the only stem rust<br />
of fi ve-needled pines. It requires an alternate<br />
host (Ribes species) <strong>for</strong> new infections<br />
to occur on pine.<br />
Early detection can be diffi cult due to<br />
very subtle symptoms during the fi rst year of<br />
infection. First, a small, yellow or red spot<br />
appears on the needle at the site of infection<br />
and eventually the needle may die as the<br />
fungus grows into the bark tissue. The newly<br />
infected bark tissue will become brown with<br />
a yellow border surrounding a section of dead<br />
needles. The stem may be slightly swollen.<br />
During the second year of infection<br />
and beyond, long, elliptical-shaped cankers<br />
develop on branches and the main trunk as<br />
the fungus advances. Cankers can eventually<br />
girdle the branch, which results in<br />
“fl agging” (Fig. 1), or they can girdle the<br />
trunk, killing all growth above the canker.<br />
Yellowish-green bark tissue may be visible<br />
around the canker. Blisters (0.25 inch,<br />
3 mm across), spores, and resin fl ow all arise<br />
from the canker area. Rodent feeding may<br />
be evident around this area as well.<br />
Biology and Life Cycle<br />
The life cycle of white pine blister rust may<br />
take 3–6 years to complete. It begins in late<br />
summer or early autumn when basidiospores<br />
from the alternate host (Ribes species)<br />
are wind and rain dispersed, entering the<br />
pine needle through the stomata (Fig. 2).<br />
Basidiospores may be carried in wind<br />
currents <strong>for</strong> up to a mile.<br />
Figure 2. Yellow/red infection site on a needle.<br />
Courtesy of USDA Forest Service Ogden<br />
Archive, Bugwood.org (#1467424)<br />
WHITE PINE<br />
BLISTER RUST<br />
Cronartium ribicola<br />
J. C. Fisch<br />
Elliptical cankers covered<br />
with yellow blisters that<br />
release infectious spores to<br />
be carried away by the wind.<br />
Courtesy of Tracey Olson,<br />
PDA<br />
WHITE PINE BLISTER RUST .................................................................................................................................................................................................................................................................... ..................................................................... 71 99
Figure 3. Elliptical cankers<br />
<strong>for</strong>med after the fi rst year of<br />
infection. Courtesy of Joseph<br />
O’Brien, USDA Forest Service,<br />
Bugwood.org (#5061066)<br />
Figure 4. Mass of powdery,<br />
yellow spores covering the<br />
surface of the canker (spring).<br />
Courtesy of Tracey Olson, PDA<br />
Figure 5. Sticky, yellow liquid<br />
exuded by a canker just be<strong>for</strong>e<br />
hardening off. Courtesy of<br />
Joseph O’Brien, USDA<br />
Forest Service, Bugwood.org<br />
(#5042098)<br />
The fungus grows into the bark tissue<br />
at a rate of 5–6 inches (12.5–15.0 cm) per<br />
year and begins to <strong>for</strong>m cankers after the<br />
fi rst year of infection (Fig. 3). In spring, 3–4<br />
years after the initial infection, pale yellow<br />
or cream-colored blisters (aecia) rupture<br />
through the bark of active cankers (Fig.<br />
4). They release powdery, yellow spores<br />
(aeciospores) that are carried in the wind<br />
over long distances to the alternate host<br />
and cause infection. The aeciospores can<br />
only infect Ribes species. After the spores<br />
are released, the cankered area on the pine<br />
remains swollen and roughened. In summer,<br />
the sticky, yellow fl uid that exudes from the<br />
site hardens and leaves small, brown-rustcolored<br />
scars (Fig. 5).<br />
On the alternate host, the aeciospores<br />
enter the stomata of the leaf during wet<br />
weather. Diffused, yellow spots become<br />
visible on the upper leaf surface soon after<br />
infection occurs. Within a few weeks, pustules<br />
<strong>for</strong>m on the leaf underside and release<br />
spores that repeatedly infect the same plant<br />
or other Ribes in the vicinity (Fig. 6). This<br />
repeating stage serves to increase the levels<br />
of inoculum. In late summer, small, brown,<br />
hairlike structures appear on the underside<br />
of the leaf. Eventually, basidiospores<br />
are produced and wind dispersed back to<br />
susceptible pines in the vicinity.<br />
Figure 6. Underside of Ribes leaf with<br />
fruiting bodies. Courtesy of Robert L.<br />
Anderson, USDA Forest Service,<br />
Bugwood.org (#0355052)<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Northeastern Pennsylvania is especially<br />
prone to white pine blister rust.<br />
• Do not plant white pine in low-lying areas<br />
where cool, moist air is likely to settle.<br />
• Do not plant white pine species if there<br />
is an abundance of alternate hosts<br />
(Ribes spp.) in the surrounding area.<br />
• Consider removing alternate host<br />
material from within 1,000 feet of<br />
white pine plantings.<br />
Preseason<br />
• Tag trees suspected to have white pine<br />
blister rust to check <strong>for</strong> blisters in<br />
mid-April.<br />
Growing Season<br />
• Scout trees <strong>for</strong> blisters in mid-April.<br />
— Randomly select at least 50 trees that<br />
are 5–10 years of age.<br />
— Look <strong>for</strong> yellow or orange blisters on<br />
branches and trunks.<br />
— Also scout tagged trees.<br />
• If 10 percent or more of sampled trees are<br />
infected:<br />
— Prune/remove cankers from trees be<strong>for</strong>e<br />
infection reaches trunk.<br />
— Control alternate host be<strong>for</strong>e August.<br />
• Remove and destroy trees with trunk<br />
infections.<br />
• Inspect trees throughout the year <strong>for</strong><br />
cankers.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Prune and destroy all branches with<br />
cankers.<br />
• Remove and destroy all alternate host<br />
plants in and around plantation within a<br />
minimum of 1,000 feet.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Apply an appropriate fungicide in late<br />
summer to protect pines from infection<br />
from basidiospores released by an alternate<br />
host.<br />
• Apply an appropriate herbicide to control<br />
the alternate host.<br />
Next Crop/Prevention<br />
• Inspect plants/nursery stock; buy from a<br />
reputable company.<br />
SHOOT AND BRANCH INJURY ............................................................................................................................................................................................................................................................ 100
Hosts<br />
In some areas, the pest is called the Sitka<br />
spruce weevil or Engelmann spruce weevil<br />
because of preference <strong>for</strong> these hosts:<br />
• Spruce<br />
• Pine<br />
• Douglas-fi r<br />
• Fir (occasionally)<br />
Damage Potential<br />
• Moderate–high (depending on stand age<br />
and species of host)<br />
Symptoms and Signs<br />
Spring<br />
• Adult weevils resting on terminal bud<br />
or on bark of previous year’s terminal;<br />
mating pairs are common; may drop to<br />
ground when approached<br />
• Pin-sized holes in the bark of terminal<br />
leader; clear droplets of sap oozing<br />
from holes; best observed on dry, sunny<br />
mornings on eastern white pine<br />
• Oval, cream-colored eggs in depression<br />
under feeding site<br />
Summer<br />
• Spongy, softened area under bark of last<br />
year’s terminal (bark may be discolored in<br />
this area)<br />
• White, legless grubs with medium brown<br />
head feeding in cambium tissue under<br />
bark of leader; multiple larvae usually<br />
present, often feeding in a ring around terminal;<br />
tissue above larvae reddish brown<br />
• By July, new terminal growth wilting<br />
or drooping, <strong>for</strong>ming characteristic<br />
shepherd’s crook; Douglas-fi r generally<br />
wilts fi rst, followed by eastern white<br />
pine; spruce damage usually last to be<br />
noticeable<br />
• Terminal dead down to second or third<br />
whorl of branches<br />
• Oval areas fi lled with shredded wood<br />
located under bark or inside the dying terminal;<br />
whitish pupae under wood shreds<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
• In late summer, round adult emergence<br />
holes about 2 ⁄25 inch (2 mm) in diameter<br />
in bark on trunk below dead terminal<br />
Throughout the Year<br />
• Tends to attack a tree in successive years,<br />
resulting in de<strong>for</strong>med or <strong>for</strong>ked trees<br />
Causes of Similar Symptoms<br />
• Frost injury<br />
• Bird damage<br />
• White pine blister rust<br />
• Eastern pine shoot borer<br />
• Zimmerman pine moth<br />
Identifi cation<br />
White pine weevil adults have long snouts<br />
typical of all weevils. Elbowed antenna<br />
with terminal knobs is another identifying<br />
characteristic <strong>for</strong> weevils. The body of<br />
white pine weevil is covered with broad,<br />
scalelike hairs that vary from medium brown<br />
to almost black. Patches of white and gold<br />
scales are noticeable on their wing coverings.<br />
In appearance, they are almost identical to<br />
eastern pine weevil, differing only in size.<br />
White pine weevil is the smaller of the two<br />
pests, measuring about ¼ inch (6–7 mm)<br />
long; males are slightly smaller than females.<br />
The larvae are white, legless, C-shaped grubs<br />
with a medium brown head and several long,<br />
silken hairs on the body.<br />
It is diffi cult to see weevils on many of<br />
the host trees because of their color, which<br />
blends well with most conifer bark. Adults<br />
are easiest to fi nd on the smooth bark of<br />
eastern white pine terminals. White pine<br />
weevil can also be diagnosed by the damage<br />
and chip cocoons under the bark of the dead<br />
terminal. No other weevil species feeds and<br />
pupates in the terminal of healthy trees.<br />
White pine weevil tends to attack a tree<br />
in successive years, resulting in de<strong>for</strong>med<br />
or <strong>for</strong>ked trees. However, young trees may<br />
be affected if found next to fi elds with high<br />
pressure. Scouting reports in Pennsylvania<br />
have shown fi elds infested at all ages.<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
Adults emerge.<br />
WHITE PINE<br />
WEEVIL<br />
Pissodes strobi (Peck)<br />
Typical “shepherd’s crook”<br />
symptomatic of white pine<br />
weevil infestation. Courtesy<br />
of PDA<br />
WHITE PINE WEEVIL .............................................................................................................................................................................................................................................................................. ................................................................... 101 71
Figure 1. Adult weevils.<br />
Courtesy of Dave Powell, USDA<br />
Forest Service, Bugwood.org<br />
(#1207040)<br />
Figure 2. Adult weevil (circled)<br />
on Colorado blue spruce.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
Figure 3. Sap droplets seeping<br />
from feeding or egg holes.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
Biology and Life Cycle<br />
White pine weevils overwinter as adults in<br />
the ground litter, or duff, under trees (Fig.<br />
1). As the weather warms in mid-March<br />
or April, adults emerge from overwintering<br />
areas. Since attacks commonly occur<br />
in subsequent years on the same tree, the<br />
overwintering site is frequently under the<br />
same tree that will be attacked in the spring.<br />
When weevils become active, they crawl up<br />
the trunk until they reach a healthy leader<br />
(Fig. 2). On warm days, they often fl y at<br />
canopy level to other trees. For 2–3 weeks,<br />
both males and females feed just below the<br />
terminal bud by chewing through the bark<br />
to make a pin-sized hole. With their strong<br />
mouthparts, they feed by scooping out the<br />
cambial layer surrounding this hole.<br />
Overwintering females were generally<br />
mated the previous fall and many retain<br />
viable sperm through the winter. However,<br />
on warm spring days, mating pairs are<br />
frequently found on terminal shoots. When<br />
eggs are mature, the female chews a fresh<br />
hole in the bark and scoops out cambial<br />
tissue to create a small egg niche. She usually<br />
deposits a single egg and seals the hole with<br />
dark brown frass be<strong>for</strong>e moving to another<br />
nearby site and repeating the process. Egg<br />
sites will have a droplet of clear sap (Fig. 3).<br />
Female white pine weevils deposit an average<br />
of 100 eggs, which hatch 6–14 days later.<br />
The whitish, oblong eggs measure 1 ⁄25 inches<br />
(1 mm) long (Figs. 4 and 5).<br />
Figure 4. Two weevil eggs sharing<br />
an egg niche. Courtesy of Sandy<br />
Gardosik, PDA<br />
Figure 5. Pale, 1-millimeter,<br />
oblong eggs under the bark and<br />
cambium layers. Courtesy of<br />
Rayanne D. Lehman, PDA<br />
When the larva emerges from the egg,<br />
it tunnels upward toward the bud be<strong>for</strong>e<br />
turning and moving downward (Fig. 6).<br />
This feeding is generally restricted to the<br />
previous year’s growth. It is common to<br />
have several larvae feeding in a ring around<br />
the terminal (Fig. 7). This effectively cuts<br />
off all fl ow of nutrients and moisture to the<br />
new growth, causing it to wilt and die. If the<br />
number of larvae is too high, competition<br />
will result in death of some larvae; if too<br />
low, the larvae may drown in pitch. When<br />
larvae drown in pitch, the terminal will<br />
survive but be de<strong>for</strong>med. Larvae from eggs<br />
deposited later in the season frequently do<br />
not survive because of lack of food.<br />
Figure 6. Larvae feeding on vascular tissue.<br />
Courtesy of Sandy Gardosik, PDA<br />
By midsummer, mature larvae are 2 ⁄5 inch<br />
(1 cm) long. Each larva creates a pupal cell<br />
under the bark or in the pith of thinner terminals.<br />
The larvae use wood strands to <strong>for</strong>m<br />
characteristic chip cocoons be<strong>for</strong>e they pupate<br />
(Fig. 8). Pupation takes several weeks,<br />
and the weevil may remain in this cell <strong>for</strong><br />
as long as 6 weeks (Fig. 9). When adults<br />
fi rst emerge, they are very pale and soft and<br />
referred to as callow adults. These callow<br />
adults can be found in the chip cocoon until<br />
their body has hardened, at which time they<br />
chew a round hole in the bark and emerge<br />
(Fig. 10). Adult emergence usually starts<br />
in mid-July and may continue into early<br />
September. Weevils will feed on branches of<br />
the host tree and mate be<strong>for</strong>e moving to the<br />
ground litter to overwinter. Most adults only<br />
overwinter one time, but reports of adults<br />
living <strong>for</strong> several years can be found in the<br />
literature.<br />
SHOOT AND BRANCH INJURY ............................................................................................................................................................................................................................................................ 102
Figure 7. Larvae feeding around<br />
the terminal. Courtesy of Daniel<br />
Herms, The Ohio State University,<br />
Bugwood.org (#1523051)<br />
Figure 8. Chip cocoon. Courtesy of<br />
Rayanne D. Lehman, PDA<br />
Figure 9. Pupae maturing in chip<br />
cocoons. Courtesy of Sandy<br />
Gardosik, PDA<br />
Figure 10. Adult weevil emerging<br />
from bark. Courtesy of Rayanne D.<br />
Lehman, PDA<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Remove any unmanaged trees that may<br />
act as pest reservoirs.<br />
• Preferred attack sites include open-grown<br />
trees in full sunlight. Terminals that are<br />
1<br />
⁄5 inch (5 mm) in diameter are preferred.<br />
Planting in partial shade may reduce<br />
attack by keeping the temperature lower,<br />
inhibiting weevil activity.<br />
Preseason<br />
• Train new tree leaders <strong>for</strong> last year’s<br />
damaged trees by using a healthy lateral<br />
branch from the previous season’s growth.<br />
• Late winter/early spring: Place modifi ed<br />
Tedder’s traps (see Appendix E) in the<br />
fi eld be<strong>for</strong>e adult weevils become active<br />
on a daily basis. Place traps in the row<br />
near trees that sustained weevil damage<br />
the year be<strong>for</strong>e (Fig. 11). Check traps<br />
regularly to monitor weevil activity (Fig.<br />
12). Traps must be baited with denatured<br />
alcohol and turpentine to be attractive to<br />
weevils.<br />
• Although trapping is the preferred detection<br />
method, do not overlook scouting on<br />
sunny days by examining leaders <strong>for</strong> adults<br />
or droplets of clear sap. Droplets will glisten<br />
in the sunlight (see Fig. 3 on p. 102).<br />
• Using binoculars is helpful <strong>for</strong> those short<br />
in stature.<br />
• When resin droplets are found, scrape<br />
away the sap to reveal if there is a circular<br />
feeding site.<br />
• Growing degree days: Adults generally<br />
become active from 7 to 58 GDDs.<br />
• Ground temperatures: After a 3-year<br />
monitoring project in Pennsylvania, it was<br />
determined that adult white pine weevils<br />
become active when ground temperatures<br />
are above 50°F. Insert a probe thermometer<br />
2 inches into the soil to collect soil<br />
temperature readings (Fig. 13). This must<br />
be monitored on the sunny side of the<br />
tree, in warmer fi elds, or on south-facing<br />
slopes fi rst.<br />
Figure 11. Tedder’s trap placed<br />
among previously damaged<br />
trees. Courtesy of Rayanne D.<br />
Lehman, PDA<br />
Figure 12. Adult weevils caught<br />
in a Tedder’s trap (plastic top<br />
has been removed). Courtesy of<br />
Sandy Gardosik, PDA<br />
Figure 13. Soil thermometer.<br />
Courtesy of Cathy Thomas, PDA<br />
WHITE PINE WEEVIL ............................................................................................................................................................................................................................................................................. 103
Growing Season<br />
• Threshold level: No threshold has been<br />
established <strong>for</strong> this pest in <strong>Christmas</strong><br />
trees. In <strong>for</strong>estry, 2–5 percent of trees<br />
damaged in the previous season is used as<br />
a treatment threshold.<br />
• Check leaders <strong>for</strong> continued feeding to<br />
determine the need <strong>for</strong> additional sprays.<br />
• When the injury becomes too severe to<br />
manage with pruning, treat the entire<br />
plantation.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• White pine weevil has several naturally<br />
occurring parasitoids, such as Lonchaeid<br />
fl ies and wasps, but they do not provide<br />
adequate control.<br />
• Birds may feed on larvae and pupae.<br />
• Rodents will consume overwintering<br />
adults.<br />
Mechanical<br />
• Prune damaged leaders as soon as wilting<br />
is detected but be<strong>for</strong>e adults emerge in<br />
mid-July. Leaders must be pruned down<br />
to healthy, green wood.<br />
• Damaged leaders should not be used to<br />
train new leaders.<br />
• Damaged tissue containing larvae or<br />
pupae should be removed from the fi eld<br />
and destroyed. Depending on the time<br />
of year and weather following pruning,<br />
adults may still be able to emerge from<br />
leaders dropped to the ground after<br />
pruning.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Chemical controls should begin as soon<br />
as the fi rst white pine weevil is trapped<br />
or feeding sites or adults are seen on<br />
terminals.<br />
• Spray applications must be made<br />
promptly. Delaying treatments results<br />
in poor control.<br />
• If a sharp drop in temperature is <strong>for</strong>ecast<br />
following the fi rst emergence of the<br />
weevils, wait to apply sprays until the<br />
next set of weevils is found in traps.<br />
• Controls should only be applied to the<br />
top third of the tree.<br />
Next Crop/Prevention<br />
• Remove any damaged or highly susceptible<br />
trees surrounding the plantation<br />
be<strong>for</strong>e planting.<br />
SHOOT AND BRANCH INJURY ............................................................................................................................................................................................................................................................ 104
STEM AND ROOT INJURY/<br />
TREE MORTALITY<br />
Entire trees may be killed by the diseases<br />
and insects on pages 107–121. Some of<br />
these may be associated with other pests<br />
that initially weaken a tree but are not<br />
capable of killing it. The combination<br />
of pests can result in rapid death of some<br />
conifers.<br />
STEM AND ROOT INJURY/TREE MORTALITY ...................................................................................................................................................................................................................................... 106
Hosts<br />
• Most species of <strong>Christmas</strong> trees<br />
• Approximately 700 different species of<br />
woody plants and some herbaceous plants<br />
Damage Potential<br />
• Low–moderate<br />
Symptoms and Signs<br />
• Reduced terminal growth<br />
• Yellowing and eventual browning of<br />
needles<br />
• Whitish resin at the base of tree<br />
• Creamy-white fans of fungus between<br />
bark and wood at the root collar<br />
• Brown to black fungal rhizomes resembling<br />
shoestrings beneath the bark, on<br />
the roots, and in the soil<br />
• Groups of tan-colored mushrooms near<br />
decaying wood in autumn<br />
• Death of young trees, often in groups<br />
Causes of Similar Symptoms<br />
• Other root and canker diseases<br />
• Pine root collar weevil<br />
• Wood borers<br />
• Bark beetles<br />
• Drought<br />
• Wet feet<br />
Identifi cation<br />
Many known species of Armillaria exist in<br />
North America and are not easily distinguishable<br />
from one another. The most common<br />
and pathogenic species to conifers are<br />
Armillaria mellea (Vahl:Fr.) P. Kumm. and<br />
A. ostoyae (Romagnosi) Herink. Other<br />
common names <strong>for</strong> Armillaria include<br />
shoestring root rot, oak fungus, honey<br />
mushroom, and honey agaric.<br />
Conifers frequently show symptoms<br />
and signs of Armillaria infection at different<br />
rates. <strong>Tree</strong>s under stress, such as during the<br />
heat of summer or drought, are more likely<br />
to become infected and show symptoms.<br />
Young trees may show symptoms and die<br />
very rapidly compared to older trees, which<br />
may show symptoms <strong>for</strong> years be<strong>for</strong>e dying.<br />
Characteristic signs of Armillaria are<br />
visible beneath the lower trunk bark and in<br />
the soil. The fungus produces long, black,<br />
stringlike strands called rhizomorphs, which<br />
can easily be mistaken <strong>for</strong> small roots. They<br />
are found between the bark and wood, on<br />
the surface of roots, and in the adjacent soil<br />
(Fig. 1). In addition, creamy-white, paperthick,<br />
fan-shaped sheets of the fungus can<br />
be seen when bark is removed at the tree<br />
base (Fig. 2) or when it is exposed in the<br />
main roots (Fig. 3).<br />
Figure 1. Black, stringlike strands, or rhizomorphs,<br />
between the bark and wood of the<br />
lower trunk and in the soil. Courtesy of Joseph<br />
O’Brien, USDA Forest Service, Bugwood.org<br />
(#5047087)<br />
Figure 2. Creamy white, paper-thin, fan-shaped<br />
sheets of mycelium under the bark near the<br />
tree’s base. Courtesy of Minnesota Department<br />
of Natural Resources Archive, Bugwood.org<br />
(#4214008)<br />
Figure 3. White fungal sheets found under<br />
the epidermis of the tree’s roots. Courtesy of<br />
Edward L. Barnard, Florida Department of<br />
Agriculture and Consumer Services, Bugwood<br />
.org (#4822096)<br />
ARMILLARIA<br />
ROOT ROT<br />
Armillaria spp.<br />
Needle yellowing and<br />
browning, a symptom of<br />
Armillaria. Courtesy of Joseph<br />
O’Brien, USDA Forest Service,<br />
Bugwood.org (#5047090)<br />
ARMILLARIA ROOT ROT ...................................................................................................................................................................................................................................................................... ................................................................... 105 107
Figure 4. Mushrooms produced<br />
by some Armillaria species near<br />
the trunk of infected trees.<br />
Courtesy of Joseph O’Brien,<br />
USDA Forest Service, Bugwood<br />
.org (#5047089)<br />
Figure 5. <strong>Tree</strong> death as a result<br />
of Armillaria infection.<br />
Courtesy of Steven Katovich,<br />
USDA Forest Service, Bugwood<br />
.org (#1479015)<br />
Diseased wood fi rst looks water soaked<br />
and light brown in color. It becomes yellowish<br />
white, spongy, and stringy over time.<br />
Prolonged decay can also cause vertical<br />
cracks in the root collar. Armillaria has a<br />
very strong mushroom odor and some species<br />
produce clusters of yellowish-brown<br />
mushrooms (associated with the rhizomorphs)<br />
near the decaying wood after a<br />
period of rain in the fall.<br />
Biology and Life Cycle<br />
Some species of Armillaria will produce<br />
mushrooms near the trunk of infected trees<br />
(Fig. 4). Though new infections can result<br />
from airborne spores released by these mushrooms,<br />
the most common means of disease<br />
spread is by underground growth of the<br />
rhizomorphs originating from an infected<br />
tree. Rhizomorph structures can survive <strong>for</strong><br />
many years on dead or dying tree roots and<br />
stumps and spread through the soil up to<br />
60 feet from the point of origin. As healthy<br />
tree roots grow through the soil, they come<br />
into contact with the rhizomorphs and<br />
mycelium. By secreting an enzyme that<br />
breaks down cell walls, the rhizomorphs and<br />
mycelium adhere to the healthy tree roots<br />
and penetrate into them. The fungus infection<br />
causes the loss of the tree’s fi ne feeder<br />
roots and results in insuffi cient water and<br />
nutrient transport to the trees, which leads<br />
to tree decline and death (Fig. 5). If the soil<br />
temperature reaches 79°F (26°C), Armillaria<br />
growth can be inhibited.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Do not plant in a recently cleared<br />
hardwood stand that had a previous<br />
problem with Armillaria.<br />
• Avoid areas surrounded by oak <strong>for</strong>est.<br />
• Avoid areas that may cause extreme<br />
tree stress such as drought or excessive<br />
moisture.<br />
Preseason<br />
• No scouting schedule or technique is<br />
available <strong>for</strong> this disease. However, when<br />
scouting <strong>for</strong> other insect pests or diseases,<br />
watch <strong>for</strong> declining trees that may be<br />
infected with Armillaria.<br />
Growing Season<br />
• Test soil and maintain proper nutrient<br />
balance.<br />
• Water when there is a drought.<br />
• Control other pests affecting trees during<br />
the season to maintain plant vigor.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Dig up and remove diseased trees, tree<br />
stumps, roots, pruning waste, and all<br />
infected wood and burn it on site.<br />
• If only a few roots are infected, remove<br />
the infected soil from midspring through<br />
late fall to expose the root collar and<br />
buttress roots to air and sun. This allows<br />
them to dry out. Be<strong>for</strong>e the fi rst heavy<br />
frost, replace the removed soil with<br />
Armillaria-free soil.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Fungicides are not recommended <strong>for</strong><br />
treatment <strong>for</strong> this disease.<br />
• Soil fumigants have been used with<br />
limited success after diseased material<br />
is removed from the soil. However, soil<br />
fumigants are highly toxic and should<br />
only be applied by a licensed pesticide<br />
professional.<br />
Next Crop/Prevention<br />
• Buy and plant disease-free stock only.<br />
STEM AND ROOT INJURY/TREE MORTALITY ...................................................................................................................................................................................................................................... 108
Hosts<br />
• Pines<br />
Damage Potential<br />
• Low (trees in fi eld) to severe<br />
(balled-and-burlapped eastern white pine)<br />
Symptoms and Signs<br />
• Needles turn yellow to red<br />
• Dying or dead parts of tree or whole dead<br />
trees<br />
• Galleries (tunnels) under loose bark<br />
• Dry, reddish-brown boring dust in bark<br />
crevices and around gallery openings on<br />
main trunk<br />
• White larvae or dark brown adult beetles<br />
in the galleries<br />
Causes of Similar Symptoms<br />
• Pine root collar weevil<br />
• Pine wilt disease<br />
• Armillaria root rot<br />
Identifi cation<br />
Bark beetles are small, cylindrical beetles<br />
about the size of a grain of rice. They are<br />
usually dark brown to black, but newly<br />
emerged adults are lighter in color. It may<br />
be diffi cult to see the head on some species.<br />
The chestnut brown bark beetle (Pityogenes<br />
hopkinsi) is about 1 ⁄ 16 inch (1.6 mm) long<br />
and is limited to eastern white pine. Ips<br />
adults are slightly larger, ranging from 1 ⁄ 8<br />
to 3 ⁄ 16 inch (3.18 to 4.76 mm) in length.<br />
Ips beetles have distinct spines on their<br />
posterior end. All species of pine are hosts<br />
<strong>for</strong> one or more species of Ips beetle. The<br />
Ips beetles are often referred to as engraver<br />
beetles because of the types of galleries they<br />
create under the bark.<br />
Larvae of these beetles are legless,<br />
creamy white, and have light brown head<br />
capsules. They may reach 3 ⁄ 16 (4.76 mm)<br />
inch long when fully grown, depending on<br />
the species.<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Biology and Life Cycle<br />
Overwintered males of both engraver<br />
(Ips) and bark (Pityogenes) beetles initiate<br />
an attack on a tree weakened by disease,<br />
environmental conditions, or other pests<br />
(Fig. 1). Each male chews a small gallery<br />
under the bark and deploys an aggregation<br />
pheromone, which attracts both sexes of<br />
the same species to the target tree (Fig. 2).<br />
In addition to bringing females <strong>for</strong> mating,<br />
the pheromone attracts more males to begin<br />
additional galleries. As a result, the beetles<br />
Figure 1. Adult bark beetle. Courtesy of Jim<br />
Stimmel, PDA, Bugwood.org (#2120093)<br />
Figure 2. Gallery of the bark beetle, Pityogenes<br />
hopkinsi. Courtesy of Rayanne D. Lehman, PDA<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
BARK AND<br />
ENGRAVER<br />
BEETLES<br />
Ips spp. and<br />
Pityogenes hopkinsi<br />
Swaine<br />
Damage from a bark beetle<br />
attack. Courtesy of PDA<br />
BARK AND ENGRAVER BEETLES ......................................................................................................................................................................................................................................................... ................................................................... 107 109
Figure 3. Bark beetle boring<br />
dust on pine trunk (symptom<br />
of beetle attack). Courtesy<br />
of Jeffrey Eickwort, Florida<br />
Department of Agriculture and<br />
Consumer Services, Bugwood<br />
.org (#5179069)<br />
Figure 4. Engraver beetle (Ips<br />
spp.) larva. Courtesy of G. Keith<br />
Douce, University of Georgia,<br />
Bugwood.org (#1879049)<br />
Figure 5. Ips spp. callow adult,<br />
larva, and pupa (from left<br />
to right). Courtesy of Roger<br />
Anderson, Duke University,<br />
Bugwood.org (#0284043)<br />
generally attack a single tree en masse (Fig.<br />
3). As the beetles enter the gallery, they<br />
introduce fungal spores, which quickly<br />
germinate. The fungus serves two purposes:<br />
(1) food <strong>for</strong> larvae, and (2) more important,<br />
it blocks resin fl ow, the tree’s main<br />
defense against bark beetle establishment.<br />
Many adults die in the attack, but the tree’s<br />
defense is eventually overcome.<br />
Females that enter the male’s initial gallery,<br />
or nuptial chamber, mate with the male<br />
and then continue chewing an egg gallery.<br />
Individual eggs are laid in niches along this<br />
gallery. When larvae emerge, they each continue<br />
a gallery radiating out from their egg<br />
niche (Fig. 4). Engraver beetles may have<br />
Y- or H-shaped galleries in the inner bark.<br />
Bark beetles, on the other hand, generally<br />
have a star-shaped gallery. Larvae pupate<br />
in the galleries (Fig. 5) and adults emerge<br />
through the bark to continue their life cycle<br />
on a different tree.<br />
Engraver beetles can complete a generation<br />
in 21–40 days under optimum conditions.<br />
How many generations are completed<br />
each year is not known. Bark beetles appear<br />
to have two main fl ight periods—one in<br />
spring and one later in summer.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Select a site <strong>for</strong> optimal pine growth.<br />
• Maintain proper spacing when planting.<br />
• Remove potential breeding material<br />
(e.g., mature pines damaged by disease,<br />
insects, wind, or other harmful causes)<br />
from the perimeter of the block.<br />
Preseason<br />
• Remove any dead or dying pines that may<br />
act as reservoirs of bark beetles.<br />
• Chip piles or tree residue can also be<br />
reservoirs.<br />
Growing Season<br />
• Pityogenes hopkinsi will attack eastern<br />
white pine under stress during and after<br />
digging (Fig. 6). Maintain adequate<br />
moisture to roots and replant as soon as<br />
possible to lessen stress.<br />
• Threshold level: Regulatory concern<br />
exists <strong>for</strong> dug trees. Consult your local<br />
state plant inspector <strong>for</strong> assistance.<br />
• Maintain tree vigor by fertilizing and<br />
irrigating as required.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• Insect parasites and predators and fungal<br />
diseases offer some control. Woodpeckers<br />
will feed on engraver (Ips) beetles,<br />
especially during the winter.<br />
Mechanical<br />
• Remove and burn, bury, chip, or debark<br />
infested parts of trees or dead trees to get<br />
rid of the insects.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Chemical control is only necessary <strong>for</strong><br />
high-valued trees in recreational or<br />
residential settings. Stressed balledand-burlapped<br />
white pine may require<br />
chemical applications during beetle<br />
fl ight periods.<br />
Figure 6. Balled-and-burlapped trees awaiting<br />
delivery—prime targets <strong>for</strong> bark beetles.<br />
Courtesy of Rayanne D. Lehman, PDA<br />
STEM AND ROOT INJURY/TREE MORTALITY ...................................................................................................................................................................................................................................... 110
Hosts<br />
• Most <strong>Christmas</strong> tree species<br />
• True fi rs, Douglas-fi r, spruce, and eastern<br />
white pine highly susceptible<br />
Damage Potential<br />
• High<br />
Symptoms and Signs<br />
• Reduced or stunted growth<br />
• Chlorotic or red-brown needles<br />
• Needle loss<br />
• Root decay<br />
• Bleeding basal cankers<br />
• Death of the tree<br />
Causes of Similar Symptoms<br />
• Armillaria root rot<br />
• Procera root rot<br />
• Bark beetles<br />
Identifi cation<br />
Phytophthora identifi cation requires laboratory<br />
analysis, but some symptoms in the fi eld<br />
should make the grower suspect this disease.<br />
<strong>Tree</strong>s that do not thrive after planting or<br />
quickly develop reddish-brown needles<br />
and exhibit dieback should be checked <strong>for</strong><br />
Phytophthora. Examine roots <strong>for</strong> symptoms<br />
of decay and absence of an extensive feeder<br />
root system (Fig. 1). Low-lying areas with<br />
poor water drainage are especially prone to<br />
root rot diseases such as Phytophthora (Fig. 2).<br />
Figure 1. Roots decay and trunk base<br />
may discolor from Phytophthora infection.<br />
Courtesy of Tracey Olson, PDA<br />
Biology and Life Cycle<br />
Phytophthora is a soilborne water mold that<br />
can spread from an infested fi eld to a new<br />
fi eld through the movement of water in the<br />
soil or on the surface. New infections can<br />
occur when the temperatures exceed 59°F<br />
(15°C). Resting spores (chlamydospores<br />
and oospores) that are capable of surviving<br />
<strong>for</strong> many years in the soil or plant are<br />
<strong>for</strong>med during cold and/or dry periods. When<br />
required temperature and moisture conditions<br />
are present, these resting spores will<br />
germinate and <strong>for</strong>m another type of sporeproducing<br />
structure called a sporangia. When<br />
mature, numerous motile, infectious spores,<br />
or zoospores, are released. Zoospores swim<br />
<strong>for</strong> up to an hour through the soil water and<br />
are attracted to the plant roots by chemicals<br />
that are produced during growth. When they<br />
come in contact with susceptible tissue, they<br />
germinate and penetrate into the roots, <strong>for</strong>m<br />
mycelium, and cause infection. Zoospores are<br />
spread farther distances from an infested fi eld<br />
to a new fi eld through the movement of fl owing<br />
surface water. It should be assumed that<br />
any plant, soil, or water that is transported<br />
from an infested fi eld is contaminated with<br />
some type of Phytophthora spore.<br />
Figure 2. Phytophthora infection often follows<br />
the slope of a hill. Courtesy of Tracey Olson,<br />
PDA<br />
PHYTOPHTHORA<br />
ROOT ROT<br />
Phytophthora spp.<br />
Severe tree browning, a<br />
symptom of Phytophthora<br />
root rot. Courtesy of Tracey<br />
Olson, PDA<br />
PHYTOPHTHORA ROOT ROT .............................................................................................................................................................................................................................................................. ................................................................... 107 111
Figure 3. Browning and loss<br />
of infected tree feeder roots.<br />
Courtesy of Edward L. Barnard,<br />
Florida Department of Agriculture<br />
and Consumer Services,<br />
Bugwood.org (#4822096)<br />
Figure 4. Sudden wilting and<br />
browning of infected tree.<br />
Courtesy of Tracey Olson, PDA<br />
As the mycelium continues to develop<br />
inside of the roots, the roots will die and<br />
turn brown (Fig. 3). The fungus will spread<br />
from the outer roots toward the larger roots,<br />
the root crown, and eventually the stem.<br />
The conductive tissue of the plants will decay<br />
and prevent fl ow of water and nutrients<br />
to the upper portion of the tree. Needles<br />
will fi rst turn chlorotic and then a reddish<br />
brown, while branches wilt (Fig. 4). This<br />
infection will lead to death of the tree.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Buy and plant healthy seedlings from a<br />
registered grower.<br />
• Do not plant in a fi eld known to be<br />
infected with Phytophthora.<br />
• Monitor seedlings be<strong>for</strong>e planting. Look<br />
<strong>for</strong> plants that show reddish-brown roots<br />
or other symptoms of root rot and do not<br />
plant these in the fi eld.<br />
• Have suspicious plants analyzed <strong>for</strong><br />
Phytophthora by a diagnostic lab.<br />
• Avoid planting Fraser fi r in areas that<br />
retain considerable moisture.<br />
• Some growers in Pennsylvania have tried<br />
mounding the soil in the rows be<strong>for</strong>e<br />
planting as a preventative strategy,<br />
though no research has been done on<br />
the effectiveness of this technique.<br />
Preseason<br />
• Do not overfertilize or overwater trees.<br />
Growing Season<br />
• Monitor fi elds <strong>for</strong> symptomatic trees. If a<br />
tree is suspected to be infected, remove<br />
the tree and the root ball from the fi eld<br />
and burn them, unless the tree is to be<br />
tested <strong>for</strong> the pathogen.<br />
• To receive confi rmation that the pathogen<br />
is Phytophthora spp., send sample to<br />
an extension laboratory or state regulatory<br />
laboratory.<br />
• <strong>Tree</strong>s that are in close proximity to known<br />
Phytophthora-infected trees should be<br />
marketed only as cut <strong>Christmas</strong> trees as<br />
soon as possible.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• No recommendations are available<br />
at this time.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• No recommendations are available<br />
at this time.<br />
Next Crop/Prevention<br />
• Replanting with susceptible hosts in<br />
known Phytophthora-infected fi elds is not<br />
recommended.<br />
• Most conifers grown in Pennsylvania<br />
are susceptible to Phytophthora spp. to<br />
some degree. Tolerance to Phytophthora<br />
has been shown with some species<br />
of Abies, most notably Turkish (A.<br />
bournmuelleriana) and Momi (A. fi rma).<br />
• Some success has occurred with using<br />
grafted Fraser fi rs to Turkish and Momi<br />
fi r rootstock in Phytophthora-infected<br />
sites, though sources <strong>for</strong> these plants are<br />
limited.<br />
STEM AND ROOT INJURY/TREE MORTALITY ...................................................................................................................................................................................................................................... 112
Hosts<br />
• Scotch, Austrian, and red pine common<br />
breeding sites; scotch pine is the least<br />
tolerant of attack; eastern white pine generally<br />
resistant due to excessive sap fl ow<br />
• Open-grown, healthy trees from 2 to 20<br />
feet (0.61–6.1 m) tall preferred; trees one<br />
inch or less in diameter at root collar are<br />
not suitable hosts<br />
• Adults readily feed on bark of eastern<br />
white, Scotch, and red pine<br />
Damage Potential<br />
• Moderate–severe<br />
Symptoms and Signs<br />
• First external symptoms: trees loose in<br />
soil, leaning, or dying (entire tree may be<br />
yellowed; infested trees may live several<br />
years be<strong>for</strong>e turning brown and dying)<br />
• Presence of blackened, pitch-soaked soil<br />
and/or white resin around the root collar<br />
• Root collar at or below soil line restricted<br />
or entire trunk broken off<br />
• Active infestations will have one or more<br />
white, legless larvae or pupae in the soil or<br />
under bark<br />
Causes of Similar Symptoms<br />
• Root rot diseases<br />
• Mechanical damage<br />
• Rodent damage<br />
Identifi cation<br />
The legless, C-shaped larvae have brown<br />
heads and whitish bodies. They closely<br />
resemble larvae of other weevil species that<br />
also attack pines. Mature larvae are ½– 2 ⁄3<br />
inch (14–17 mm) in length. A good fi eld<br />
identifi cation tip <strong>for</strong> this pest is the location<br />
of the larvae. Pine root collar immatures<br />
are only found in the root collar area below<br />
ground level. To locate larvae, remove soil<br />
down to the fi rst root fl are. They may be<br />
in the cambial layer of the host or in the<br />
surrounding pitch-soaked soil. Occasionally,<br />
pitch tubes extending from the root collar<br />
into the soil will be found.<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
Pine root collar weevils never pupate in<br />
a typical chip cocoon, unlike other conifer<br />
weevil pests. Instead, they create their pupal<br />
cell surrounded by soil or pupate in their<br />
gallery in the bark of the root collar area.<br />
Adults are typical weevils with elongated<br />
snouts and elbowed antennae. They<br />
are dark brown with small patches of fi ne,<br />
pale hairs on the wing covers. The adults<br />
resemble pales weevil adults but are slightly<br />
larger, ranging from 1 ⁄3 to ½ inch long (10.0–<br />
12.5 mm) (Fig. 1). Consult a plant inspector<br />
or entomologist to confi rm identifi cation.<br />
Figure 1. Adult pine root collar weevil (left);<br />
pales weevil (right). Courtesy of Jim Stimmel,<br />
PDA<br />
Biology and Life Cycle<br />
Only adults are active above the soil line,<br />
where they will feed on tender bark of pine<br />
twigs. They are nocturnal feeders and prefer<br />
to feed on the underside of lower branches.<br />
Adults also move from tree to tree at night<br />
but crawl on the ground since they are weak<br />
fl iers. They must be careful of their movements<br />
during the summer because a soil<br />
surface temperature greater than 104°F will<br />
kill them. Adults also feed with the larvae<br />
on the inner bark of the root crown.<br />
The life cycle of pine root collar weevil<br />
is completed in two seasons. In early May,<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
PINE ROOT<br />
COLLAR WEEVIL<br />
Hylobius radicis<br />
Buchanan<br />
Dying tree with pine root<br />
collar weevil infestation.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
PINE ROOT COLLAR WEEVIL ............................................................................................................................................................................................................................................................... ................................................................... 107 113
Figure 2. Egg laying and larval<br />
feeding within the root collar<br />
just below the soil. Courtesy of<br />
Rayanne D. Lehman, PDA<br />
Figure 3. Larva feeding on the<br />
root collar. Courtesy of James B.<br />
Hanson, USDA Forest Service,<br />
Bugwood.org (#1394091)<br />
Figure 4. Excessive pitch in the<br />
soil surrounding the root collar<br />
resulting from larval feeding.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
Figure 5. Pupa in soil near the<br />
root collar. Courtesy of Sandy<br />
Gardosik, PDA<br />
young overwintering adults emerge. Mating<br />
and egg laying occur at the base of the tree<br />
during the day and egg laying may continue<br />
into September. Eggs are deposited<br />
in feeding wounds made by an adult in the<br />
inner bark of the root collar (Fig. 2) or a<br />
few centimeters away from a root in the soil.<br />
Two weeks later, the eggs hatch and young<br />
larvae start feeding on the inner bark of the<br />
root collar area (Fig. 3). This causes pitch<br />
(or sap) to fl ow into the surrounding soil,<br />
eventually reaching 15–20 inches (40–50<br />
cm) from the trunk (Fig. 4). Larvae create<br />
tubes in this pitch-soaked soil to drain excess<br />
pitch away from the wound area. They<br />
may also use the pitch tubes <strong>for</strong> molting and<br />
pupating.<br />
The majority of larvae overwinter in the<br />
root collar as third and last instars. However,<br />
with the length of egg laying, early<br />
eggs may produce adults in the same season.<br />
Overwintered larvae become active the<br />
following spring, complete development,<br />
and pupate in late June to mid-August<br />
(Fig. 5). The new adults begin to emerge in<br />
late summer and feed on pine bark be<strong>for</strong>e<br />
overwintering in litter under the tree or<br />
under the bark. These adults emerge the following<br />
spring to mate and lay eggs and may<br />
overwinter a second winter. Second-year<br />
adults will emerge in the spring to continue<br />
feeding, mate, and lay eggs be<strong>for</strong>e dying in<br />
early summer.<br />
All life stages of this weevil can be found<br />
during the growing season. During the winter,<br />
only large larvae and possibly pupae and<br />
adults will be present. Vigorously growing<br />
trees respond to larval feeding injuries by<br />
producing massive amounts of resin under<br />
the bark at and near the site of injury (Fig.<br />
6). Affected trees continue to live an average<br />
of 2–4 years after the initial attack (Fig. 7).<br />
Figure 6.<br />
Infected root<br />
collar soaked<br />
with resin.<br />
Courtesy of<br />
Steven<br />
Katovich,<br />
USDA Forest<br />
Service,<br />
Bugwood.org<br />
(#1199001)<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Establish fi elds at least one mile away from<br />
any known infestations.<br />
• Plant least resistant varieties such as<br />
Scotch pine in small, single-species<br />
blocks.<br />
• Interplant pines only with fi r, spruce, or<br />
deciduous trees; do not interplant Scotch<br />
pine with other pines.<br />
Preseason<br />
• Examine off-color trees <strong>for</strong> symptoms.<br />
Rocking trees by hand will reveal any<br />
loose in the soil. Follow up with an<br />
examination of the root collar area <strong>for</strong><br />
other symptoms.<br />
Growing Season<br />
• Threshold level: No threshold has been<br />
established.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Figure 7. Mixed pine stand with varying<br />
degrees of infestation; damage is worst along<br />
the block’s edge where trees are stressed<br />
from grass competition. Courtesy of Steven<br />
Katovich, USDA Forest Service, Bugwood.org<br />
(#1199002)<br />
STEM AND ROOT INJURY/TREE MORTALITY ...................................................................................................................................................................................................................................... 114
Control Options<br />
Biological<br />
• Use of parasites is not practical because<br />
larvae are below the soil and usually<br />
protected by pine pitch.<br />
Mechanical<br />
• To increase soil temperature and there<strong>for</strong>e<br />
decrease existing populations and lessen<br />
the risk of infestations, butt-prune trees a<br />
minimum of 12 inches (30.48 cm) high.<br />
• Remove leaf litter from under the tree,<br />
allowing the sun to heat and dry the soil.<br />
• Remove 1–2 inches (2.54–5.08 cm) of soil<br />
away from the trunk base, aiding in creating<br />
an undesirable environment <strong>for</strong> adult<br />
activity and egg laying.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• Insecticide applications are only effective<br />
against adults when they are active at the<br />
base of the host tree.<br />
— Make an application in mid-May to<br />
control adults be<strong>for</strong>e egg laying.<br />
— Make a second application in mid-<br />
August to mid-September to kill newly<br />
emerging adults.<br />
— Both applications are required <strong>for</strong><br />
successful control.<br />
• Apply only registered products <strong>for</strong> your<br />
state. Follow the directions on the label<br />
thoroughly.<br />
PINE ROOT COLLAR WEEVIL ................................................................................................................................................................................................................................................................ 115
PINE WILT<br />
DISEASE<br />
Caused by pinewood<br />
nematode,<br />
Bursaphelenchus<br />
xylophilus<br />
(Steiner and Buhrer)<br />
(Nickle)<br />
Dead tree with<br />
reddish-brown needles still<br />
attached. Courtesy of<br />
A. Steven Munson, USDA<br />
Forest Service, Bugwood.org<br />
(#1470133)<br />
Host<br />
• Pines, particularly Scotch pine<br />
Damage Potential<br />
• Moderate–severe<br />
Symptoms and Signs<br />
• Considerable decrease of resin fl ow from<br />
wounds<br />
• Needles turn yellow then reddish brown<br />
through growing season (wilting)<br />
• Needles may remain attached <strong>for</strong> several<br />
months following sudden death of tree<br />
• Wood of affected trees dries out and<br />
completely lacks resin<br />
Causes of Similar Symptoms<br />
• Wood borers and bark beetles<br />
• Diplodia tip blight<br />
• Atropellis canker<br />
Identifi cation<br />
This pest can only be identifi ed using a<br />
microscope. Check dying trees <strong>for</strong> symptoms<br />
and, if pine wilt disease is suspected, have<br />
samples examined by a pest specialist to<br />
determine if nematodes are present in the<br />
wood.<br />
Biology and Life Cycle<br />
Pinewood nematodes spread from infected<br />
pines to healthy or stressed pines in the<br />
spring through contaminated pine sawyer<br />
beetles (Fig. 1). These long-horned beetles<br />
acquire the nematode from infested trees<br />
during their development within the tree.<br />
When new adult beetles emerge in spring,<br />
they transfer nematodes to healthy trees<br />
through feeding or to diseased trees during<br />
egg-laying activities.<br />
Figure 1. Pine sawyer beetle is a vector of<br />
pinewood nematode. Courtesy of L. D. Dwinell,<br />
USDA Forest Service, Bugwood.org (#4387008)<br />
The nematodes have two phases. The<br />
propagative phase occurs in the sapwood of<br />
infested trees and includes six stages from<br />
egg to adult. A single generation can be<br />
completed in as few as 5 days under ideal<br />
conditions. This enables the nematodes to<br />
rapidly develop extremely high populations<br />
(Fig. 2). As they feed in the resin ducts and<br />
cambial tissues, the tree’s water-conducting<br />
system fails, causing rapid wilt under dry<br />
conditions (Fig. 3).<br />
Figure 2. Pinewood nematode feeding on resin<br />
ducts in pines. Courtesy of USDA Forest Service<br />
Rocky Mountain Region Archive, Bugwood.org<br />
(#1442033)<br />
Figure 3. Severe wilt caused by a failure of the<br />
water-conducting system from nematode feeding.<br />
Courtesy of USDA Forest Service North<br />
Central Research Station Archive, USDA Forest<br />
Service, Bugwood.org (#1406272)<br />
STEM AND ROOT INJURY/TREE MORTALITY ...<br />
...................................................................................................................................................................................................................................... 116
The second phase of the nematode life<br />
cycle is the dispersal phase. This only occurs<br />
in the late stage of tree infection when<br />
pine sawyer pupae are present. Under these<br />
conditions, immature nematodes develop<br />
into a nonfeeding stage that attaches to the<br />
pupal cell of the beetle (Fig. 4). When the<br />
adult beetles emerge, the nematodes will<br />
have contaminated their respiratory system<br />
and are carried with them to new host trees.<br />
Nematodes leave the beetle and enter the<br />
shoots of a new tree through the wounds<br />
created by beetle-feeding activity. Within<br />
48 hours of introduction to a new, healthy<br />
host, the nematodes have matured into<br />
reproducing adults.<br />
Pinewood nematodes feed on epithelial<br />
cells and resin ducts of healthy trees. They<br />
also may feed on blue-stain fungi in dead<br />
and dying trees to sustain or build population<br />
levels (Fig. 5). The fungus feeding<br />
cycle is the most common stage in North<br />
America.<br />
Figure 4. Nematodes entering the pine sawyer<br />
pupae will be transferred to a new host when<br />
the pine sawyer adults emerge. Courtesy of<br />
USDA Forest Service North Central Research<br />
Station Archive, Bugwood.org (#1406269)<br />
Figure 5. Pinewood nematodes<br />
feeding on blue stain fungi found in<br />
weak and dying trees. Courtesy of<br />
USDA Forest Service North Central<br />
Research Station Archive, Bugwood<br />
.org (#1406276)<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Avoid planting on traditionally dry sites.<br />
• Do not plant susceptible pines in areas<br />
where mean summer temperatures are<br />
above 68°F.<br />
Preseason<br />
• No recommendations are available<br />
at this time.<br />
Growing Season<br />
• Maintain tree vigor through periodic<br />
fertilization and irrigation during times of<br />
dry weather.<br />
• Any trees exhibiting symptoms should be<br />
analyzed by a diagnostic lab to determine<br />
presence of pine wilt nematode.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• No recommendations are available<br />
at this time.<br />
Mechanical<br />
• Remove and destroy (burn, bury, and/or<br />
chip) trees to help prevent spread of pest<br />
to nearby, healthy pines in the spring,<br />
be<strong>for</strong>e beetle emergence.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• No recommendations are available<br />
at this time.<br />
Next Crop/Prevention<br />
• Buy and plant disease-free stock only.<br />
PINE WILT DISEASE ............................................................................................................................................................................................................................................................................... 117
WHITE GRUBS<br />
(MAY, JUNE,<br />
AND JAPANESE<br />
BEETLES)<br />
Phyllophaga sp.,<br />
Polyphylla sp.,<br />
Popillia japonica<br />
Newman<br />
White grub damage to<br />
seedlings. Courtesy of<br />
Jim Stimmel, PDA<br />
Hosts<br />
• All conifers<br />
Damage Potential<br />
• Moderate–severe on seedlings<br />
Note: Balled-and-burlapped trees are<br />
regulated <strong>for</strong> the Midwest and western<br />
United States and parts of Canada,<br />
according to the U.S. Japanese Beetle<br />
Harmonization plan. For more in<strong>for</strong>mation,<br />
refer to nationalplantboard.org/docs/<br />
jbcolumn.pdf.<br />
Symptoms and Signs<br />
• Seedlings and young conifer saplings<br />
discolored (reddish brown) in late summer<br />
to early fall, leading to eventual death<br />
• Lateral and taproots chewed off or girdled<br />
• Small holes, ¼– 1 ⁄3 inch (6–8 mm) in<br />
diameter, in soil surface<br />
Causes of Similar Symptoms<br />
• Drought stress<br />
• Phytophthora root rot<br />
• Pales or eastern pine weevil feeding<br />
Identifi cation<br />
Larvae of several species of scarab beetles,<br />
including the Japanese beetle, feed on roots<br />
of conifer seedlings and transplants. Collectively,<br />
the larvae are referred to as white<br />
grubs and are similar in appearance. Mature<br />
larvae are 1–2 inches (25–55 mm) in<br />
Figure 1. Adult Japanese beetle feeding<br />
on conifers (rare). Courtesy of Sandy<br />
Gardosik, PDA<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
length. These C-shaped, grayish-white grubs<br />
have tan heads and visible jointed legs.<br />
The posterior end of the grub is enlarged<br />
and frequently darkened. Most species have<br />
sparse yellow hairs on the body. All white<br />
grubs have a set of minute spines around the<br />
anus. The location of these spines, known<br />
as the raster pattern, is used to identify some<br />
species.<br />
Adults are dark brown to black beetles<br />
up to an inch (25 mm) in length. The common<br />
Japanese beetle adult is more colorful<br />
and familiar to anyone who gardens. Adult<br />
Japanese beetles have rarely been reported<br />
to feed on tender bark during a serious<br />
outbreak (Fig. 1).<br />
Biology and Life Cycle<br />
Scarab larvae, or white grubs, overwinter<br />
in the soil (Fig. 2). In spring, they move to<br />
the upper 4 inches of soil and resume feeding<br />
on the roots of almost every plant they<br />
encounter. When fully grown, these grubs<br />
create soil-encrusted cells be<strong>for</strong>e pupating<br />
near the soil surface. Adults begin to emerge<br />
in May and June. After feeding and mating,<br />
females deposit eggs just below the soil surface.<br />
As larvae emerge from the eggs, they<br />
immediately begin to burrow underground.<br />
Adequate soil moisture is required <strong>for</strong> survival,<br />
and drought periods will signifi cantly<br />
decrease the population due to desiccation<br />
of young larvae.<br />
Figure 2. June beetle larva. Courtesy of<br />
Steven Katovich, USDA Forest Service,<br />
Bugwood.org (#2121096)<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
STEM AND ROOT INJURY/TREE MORTALITY ...<br />
...................................................................................................................................................................................................................................... 118
Through the summer, the larvae feed<br />
in the top 4 inches of soil. They may travel<br />
considerable distances in search of plant<br />
roots on which to feed. Their strong mouthparts<br />
are capable of cutting off taproots of<br />
smaller seedlings. When grubs feed on conifer<br />
seedlings, the roots are consumed and<br />
the tree may be girdled just under the soil<br />
surface (Fig. 3). In beds of young seedlings,<br />
the plants may appear to be pulled deeper<br />
into the soil as the grubs feed. From time<br />
to time, the grubs emerge to the surface to<br />
deposit feces outside their burrow. In this<br />
process, they leave behind round holes in<br />
the surface that resemble puncture holes<br />
from a pencil.<br />
As soil temperatures decrease in fall,<br />
grubs move lower in the soil. They burrow<br />
8–10 inches (20.32–25.40 cm) below the<br />
soil surface to remain below the frost line<br />
overwinter. Japanese beetles only require 1<br />
year to complete a life cycle, but members<br />
of the genera Phyllophaga and Polyphylla<br />
require up to 4 years.<br />
Damage in seedling beds can occur as<br />
early as the fi rst year after germination.<br />
However, in transplants, the most severe<br />
white grub damage generally occurs 2–3<br />
years after fi eld planting. This is when the<br />
grubs are mature and capable of severing<br />
the taproots. Plantings that were previously<br />
in pasture or grass are the most susceptible<br />
and, generally, the grubs were present be<strong>for</strong>e<br />
the trees were planted.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Corn fi elds are known reservoirs <strong>for</strong><br />
Japanese beetles. Conifer fi elds planted<br />
adjacent to these could be more susceptible<br />
to grub damage.<br />
• Evaluate the present white grub population<br />
in early fall or spring be<strong>for</strong>e planting.<br />
• Dig a 10-foot-long (3.05 m) furrow. If<br />
one grub per 10 feet of furrow is found,<br />
consider treating be<strong>for</strong>e planting the<br />
following year.<br />
• Dig several square-foot holes and sift<br />
through soil to check <strong>for</strong> grubs. If more<br />
than one grub per hole is present, a<br />
preplanting treatment is recommended.<br />
Preseason<br />
• No recommendations are available<br />
at this time.<br />
Growing Season<br />
• Threshold levels are <strong>for</strong> grubs in soil prior<br />
to planting. See Plantation Establishment<br />
above.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological/Biorational<br />
• The bacterial powder Bacillus popillae<br />
causes a milky spore disease specifi c to<br />
Japanese beetle. Application to grassy<br />
areas in early summer or late fall allows<br />
grubs to ingest the bacteria, which will<br />
kill them be<strong>for</strong>e spring.<br />
• Pathogenic fungi, nematodes, and protozoans<br />
may help decrease grub populations.<br />
• The parasitic wasps Tiphia popilliavora<br />
(Rohwer) and Tiphia vernalis (Rohwer)<br />
are parasites of Japanese beetle and some<br />
other white grubs.<br />
• Birds and toads consume many beetles in<br />
addition to a small quantity of grubs.<br />
• Moles, shrews, and skunks feed heavily on<br />
grubs in the soil.<br />
Mechanical<br />
• For low numbers of grubs, hand removal<br />
from tilled soil may be helpful.<br />
• For new seedling beds, till or disk several<br />
times in April–May or September to<br />
injure larvae in soil and expose them to<br />
parasite and predators.<br />
• Some states recommend maintenance<br />
of groundcover to offer alternate food<br />
sources to grubs in the plantation.<br />
Chemical<br />
• Apply a registered insecticide with a<br />
stomach poison to grassy areas in the<br />
spring (March–mid-May) or fall<br />
(September–November) to control grubs.<br />
• Apply a registered insecticide as a preventative<br />
drench at the base of the plant,<br />
directed at the root ball/zone.<br />
• Root dip may be hard on trees, so in-fi eld<br />
treatment is preferred.<br />
Figure 3. Seedling with grub<br />
feeding damage (left) and<br />
healthy seedling (right).<br />
Courtesy of James Solomon,<br />
USDA Forest Service,<br />
Bugwood.org (#3066089)<br />
WHITE GRUBS (MAY, JUNE, AND JAPANESE BEETLES) ...................................................................................................................................................................................................................... 119
ZIMMERMAN<br />
PINE MOTH<br />
Dioryctria zimmermani<br />
(Grote)<br />
Pitch mass, a symptom of<br />
Zimmerman pine moth,<br />
located at the junction of<br />
a lateral branch and main<br />
stem. Courtesy of Whitney<br />
Cranshaw, Colorado State<br />
University, Bugwood.org<br />
(#1325082)<br />
Hosts<br />
• All pines, especially Austrian and Scotch<br />
• Occasionally found on Douglas-fi r and<br />
spruce<br />
Damage Potential<br />
• Moderate–severe<br />
Symptoms<br />
• Popcornlike, white to pinkish pitch mass<br />
on trunk, often at a branch whorl or on<br />
shoots near the terminal leader; transplants<br />
and young trees may have pitch<br />
mass closer to soil line<br />
• Reddish, sawdustlike frass mixed with<br />
pitch at entrance sites of bore holes<br />
• Broken leader or lateral branches; dead or<br />
dying branches on upper half of tree<br />
• Sawdust collecting on lateral branches<br />
and in webbing on the tree in early<br />
summer<br />
• Empty pupal case protruding from wound<br />
in main trunk<br />
Causes of Similar Symptoms<br />
• White pine weevil<br />
Identifi cation<br />
Adults, eggs, and young larvae of Zimmerman<br />
pine moths are very rarely seen. Their<br />
coloration and elusive habits help them<br />
avoid detection. Field identifi cation is usually<br />
through the presence of a pitch mass<br />
on the main trunk. This mass is popcornlike<br />
and yellow to pinkish in color. Frass is<br />
usually mixed in with the pitch. When the<br />
glob of pitch is removed, a small, elongate/<br />
oval hole is usually found in the trunk. In<br />
an active infestation, the pitch mass will be<br />
somewhat pliable and soft. Hardened masses<br />
are generally from larval activity in the<br />
previous season.<br />
From May to August, a single larva<br />
may be found in a short gallery in an active<br />
infestation. Zimmerman pine moth larvae<br />
are pinkish to greenish and up to an inch<br />
(25 mm) long when mature. They have a<br />
dark brown head and numerous dark spots<br />
Calendar of Activities<br />
Symptoms<br />
Monitor<br />
Mechanical<br />
Control<br />
Spray Control<br />
on the body. The ¾-inch-long (18-mm)<br />
pupa may be found at the exit of the feeding<br />
tunnel from mid-July to late August.<br />
Biology and Life Cycle<br />
Zimmerman pine moths overwinter in the<br />
bark of the tree trunk as small caterpillars<br />
that emerged from eggs in late summer or<br />
early fall. The newly hatched caterpillars<br />
do not feed but quickly move to nearby<br />
protected sites under bark scales or in<br />
crevices below a main lateral branch. They<br />
chew small cavities in the bark and cover<br />
themselves with a silken tent, or hibernaculum,<br />
in which they will overwinter.<br />
When Scotch pine terminal growth starts<br />
the following April, the larvae leave their<br />
overwintering site and chew into the inner<br />
bark around the junction of a lateral branch<br />
and main trunk. Other favored entrance<br />
sites include wounds and galls caused by<br />
pine-pine or pine-oak gall. Small amounts<br />
of sawdust may be seen clinging to branches<br />
and in spider webs at this time.<br />
As the larvae feed in the phloem tissue,<br />
sap fl ows into the tunnel. Larvae push<br />
this sap—mixed with their frass and other<br />
debris—out of the tunnel, creating the characteristic<br />
pitch mass (Fig. 1). Occasionally,<br />
larvae will leave one feeding site and create<br />
a second site nearby. This feeding weakens<br />
the tree branches and often causes breakage<br />
Figure 1. Pitch<br />
mass made up<br />
of sap, frass,<br />
and tree debris<br />
that has<br />
been pushed<br />
out of the<br />
feeding tube<br />
by the larva.<br />
Courtesy of<br />
Rayanne D.<br />
Lehman, PDA<br />
Jan. Feb. March April May June July Aug. Sept. Oct. Nov. Dec.<br />
STEM AND ROOT INJURY/TREE MORTALITY ... ...................................................................................................................................................................................................................................... 120
Figure 2. Larva<br />
feeding within<br />
the trunk. Courtesy<br />
of Rayanne D.<br />
Lehman, PDA<br />
Figure 3. Weakened branch junction due<br />
to Zimmerman pine moth feeding damage.<br />
Courtesy of Minnesota Department of Natural<br />
Resources Archive, Bugwood.org (#4212056)<br />
(Figs. 2 and 3). Feeding continues through<br />
the summer and the larvae are mature by<br />
August (Fig. 4).<br />
The larvae pupate inside their feeding<br />
tunnel, close to the entrance site, or in<br />
the soft pitch mass (Fig. 5). Adult moths<br />
emerge 2–3 weeks later, generally in late<br />
July through August. The short-lived adults<br />
are weak, nocturnal fl iers that spend most<br />
of their time resting on the bark of the host<br />
tree (Fig. 6). The coloration provides excellent<br />
camoufl age and they are rarely seen. In<br />
late summer, each mated female deposits up<br />
to 80 eggs on the bark of the main trunk.<br />
The eggs hatch in early fall. One generation<br />
occurs each year.<br />
Monitoring and <strong>Management</strong><br />
Strategies<br />
Plantation Establishment<br />
• Plant resistant varieties of Scotch pine,<br />
especially short-needled ones from<br />
Greece, Turkey, and others from south<br />
and west Eurasia.<br />
• Observe proper planting practices,<br />
including depth of transplants. Planting<br />
too deep will lead to health issues.<br />
Preseason<br />
• Remove and destroy heavily damaged<br />
trees. Zimmerman pine moth frequently<br />
reinfests trees; removal of these “brood”<br />
trees may lessen damage to healthy trees.<br />
Growing Season<br />
• Inspect blocks on a regular basis<br />
throughout the growing season.<br />
• Look <strong>for</strong> pitch masses on the main trunk<br />
or near terminal leader. In young trees,<br />
masses maybe closer to soil line.<br />
• Avoid mechanical damage to trunks that<br />
may increase susceptibility to this pest.<br />
• Chemically or mechanically control pine<br />
galls to decrease attractive larval feeding<br />
sites.<br />
• Growing degree days: Larvae emerge from<br />
overwintering sites at 121–246 GDDs.<br />
• Threshold level: No threshold has been<br />
established.<br />
• At the end of the season, evaluate results<br />
and update records.<br />
Control Options<br />
Biological<br />
• Many larval and Trichogramma egg parasitoids<br />
have been identifi ed in southern<br />
Michigan. Native parasitic wasps infested<br />
up to 57 percent of larvae and 45 percent<br />
of eggs in the study area.<br />
Mechanical<br />
• Cut out areas with pitch masses on<br />
the main trunk with a pocket knife or<br />
shearing tool and use a thin wire to<br />
destroy larvae in gallery.<br />
• Hand-prune and destroy occasional<br />
injured shoots.<br />
• Remove and destroy (by burning or<br />
chipping) infested trees by early July<br />
be<strong>for</strong>e adults emerge.<br />
Biorational<br />
• No recommendations are available<br />
at this time.<br />
Chemical<br />
• When larvae are abundant or repeatedly<br />
attacking the main trunk, apply a registered<br />
insecticide in early April–early May<br />
as the weather warms.<br />
— Emerging larvae are the most vulnerable<br />
to pesticides be<strong>for</strong>e boring under<br />
bark.<br />
— Use enough nozzle pressure and water<br />
to drench branch and trunk bark.<br />
Figure 4. Larva with pink-green<br />
body, dark brown head, and<br />
small, dark spots. Courtesy of<br />
Rayanne D. Lehman, PDA<br />
Figure 5. Pupae close to the exit<br />
of the feeding tunnel. Courtesy<br />
of Rayanne D. Lehman, PDA<br />
Figure 6. Adult moth with<br />
coloration that allows it to<br />
blend well with the tree bark.<br />
Courtesy of Whitney Cranshaw,<br />
Colorado State University,<br />
Bugwood.org (#1246008)<br />
ZIMMERMAN PINE MOTH .................................................................................................................................................................................................................................................................... 121
OTHER TYPES OF<br />
DAMAGE TO CONIFERS<br />
Other factors beyond disease, insect, and<br />
mite pests can cause signifi cant damage<br />
to <strong>Christmas</strong> trees that may resemble pest<br />
damage. These factors fall into two broad<br />
categories. The abiotic factors include<br />
those not caused by living organisms as<br />
well as chemical, environmental, and<br />
mechanical damage. The biotic factors<br />
are those caused by several vertebrate<br />
pests. Both of these types of damage are<br />
discussed on pages 123–129.<br />
OTHER TYPES OF DAMAGE TO CONIFERS .......................................................................................................................................................................................................................................... 122
Chemical Damage<br />
Chemical damage can result from pesticide<br />
applications made during the growing<br />
season. There are varied reasons <strong>for</strong> this type<br />
of damage, such as mixing incompatible<br />
materials, using the wrong concentration,<br />
applying to tender foliage, overlooking drift<br />
or overspray, using equipment that has previously<br />
contained an herbicide, or even using a<br />
material that is not labeled <strong>for</strong> use on <strong>Christmas</strong><br />
trees (Figs. 1 and 2). The root cause of<br />
most chemical damage is applicator error.<br />
Figure 1.<br />
Glyphosate<br />
damage to spruce.<br />
Courtesy of<br />
Rayanne D.<br />
Lehman, PDA<br />
Figure 2. Distortion of spruce foliage due to<br />
herbicide application. Courtesy of Ricky Bates,<br />
Penn State<br />
Figure 3. Chemical burn all along the row on<br />
lower portion of the trees typical of improper<br />
sprayer adjustment. Courtesy of Rayanne D.<br />
Lehman, PDA<br />
Typically, symptoms of chemical damage<br />
follow the path of application through the<br />
fi eld (Fig. 3). If drift or overspray is thought<br />
to be the culprit, damage would only occur<br />
in the portion of the fi eld closest to the<br />
intended spray target (Fig. 4). If the damage<br />
is throughout the fi eld, mixing error, use of<br />
the wrong material, or equipment contamination<br />
may be the cause. Damage limited<br />
to new growth may result if an application<br />
is made be<strong>for</strong>e the growth has hardened<br />
off. These types of damage appear quickly<br />
during the growing season. During the rest<br />
of the year, damage appears at a slower rate.<br />
Some insecticide oils can burn new foliage,<br />
but most chemical injuries to trees are from<br />
herbicide applications (Figs. 5 and 6).<br />
Figure 4.<br />
Chemical<br />
burn due to<br />
improper<br />
sprayer<br />
adjustment.<br />
Courtesy of<br />
Rayanne D.<br />
Lehman, PDA<br />
Figure 5. Damage from simazine application.<br />
Courtesy of Ricky Bates, Penn State<br />
Figure 6. <strong>Tree</strong>s exhibiting<br />
glyphosate burn on left;<br />
healthy trees on right. Courtesy<br />
of Ricky Bates, Penn State<br />
CHEMICAL DAMAGE ............................................................................................................................................................................................................................................................................ 123
Figure 1. Air pollution damage<br />
on eastern white pine.<br />
Courtesy of USDA Forest<br />
Service Region 8 Archive,<br />
Bugwood.org (#1505030)<br />
Figure 2. Needle discoloration<br />
and drop as a result of air<br />
pollution. Courtesy of USDA<br />
Forest Service Northeastern<br />
Area Archive, Bugwood.org<br />
(#1398007)<br />
Environmental Damage<br />
Air Pollution<br />
Air pollution has been shown to affect all<br />
conifers, with each species responding differently.<br />
In general, eastern white pine is<br />
the most susceptible, but spruce may be the<br />
most sensitive in industrial areas.<br />
A tree’s response to airborne pollutants<br />
is affected by temperature, light intensity,<br />
water availability, and soil nutrients.<br />
Airborne pollutants may cause a decrease<br />
in photosynthesis, resulting in carbohydrate<br />
shortage in older needles. This leads to a<br />
decrease in new needle development and<br />
growth. <strong>Tree</strong>s attempt to conserve energy<br />
when exposed to high levels of air pollution<br />
and may undergo early needle drop or<br />
senescence (Figs. 1 and 2). <strong>Tree</strong>s growing in<br />
shallow or poorly drained soils may already<br />
be near the limits of their environmental<br />
requirements and would be predisposed to<br />
stress from air pollution. <strong>Tree</strong>s growing in<br />
better soils are more susceptible to damage<br />
in late summer due to the high rates of<br />
photosynthesis at that time. Decreased<br />
radial growth (diameter expansion) often<br />
results, but tree death may also occur.<br />
Some experiments have shown decreases<br />
in tree hardiness and frost resistance due to<br />
an increase in ozone levels caused by pollution.<br />
However, others have shown that high<br />
ozone levels did not affect frost resistance.<br />
On the positive side, a few studies have<br />
shown that an increase in carbon dioxide<br />
(CO 2 ) improves photosynthesis and rootto-shoot<br />
ratio in fi eld-grown plants, provided<br />
they are of optimal nutrition. Some<br />
naturally occurring antioxidants have been<br />
identifi ed in trees. These compounds may<br />
reverse some of the effects of air pollution.<br />
From available research, it has been shown<br />
that conifers have a higher tolerance to<br />
pollution during their dormant period than<br />
when actively growing.<br />
Suggestions <strong>for</strong> limiting damage from<br />
air pollution include pruning dead shoots,<br />
culling dying trees to prevent other pests,<br />
avoiding planting near a source of pollution,<br />
and planting air-pollution-resistant species.<br />
Drought<br />
Drought damage is the result of trees needing<br />
more moisture than what is available<br />
in the soil. It affects all ages of trees, but<br />
new transplants are the most vulnerable.<br />
Symptoms generally start at the top of<br />
the tree and continue downward and may<br />
include the loss of needles, fading or yellowing<br />
of needles, and overall slow growth.<br />
During extreme drought, tree mortality may<br />
occur (Fig. 3). The effects of drought may<br />
be from one growing season of signifi cantly<br />
decreased moisture or from several seasons<br />
of below-average rainfall. Drought also leads<br />
to other problems, especially insect pests<br />
and diseases.<br />
Heat and Sunscald<br />
Extreme heat from intense sunlight can<br />
damage all <strong>Christmas</strong> trees, especially<br />
during bud break. Heat damage generally<br />
occurs on the southern or southwestern exposure,<br />
where needles quickly turn reddish<br />
brown, causing the entire tree to appear<br />
burned (Fig. 4).<br />
Sunscald is caused when young, thinbarked<br />
trees are exposed to concentrated<br />
sunlight and the cambium under the bark<br />
dies. It may also coincide with drought<br />
conditions. The bark will take on a coppery<br />
brown color during summer and branches<br />
above the site may die. Eventually, the bark<br />
may slough off. Damage may be limited to<br />
Figure 3. A whole block of dead seedlings as a<br />
result of drought. Courtesy of PDA<br />
Figure 4. Foliage burn on the southern side of<br />
the tree caused by intense sunlight. Courtesy of<br />
Cathy Thomas, PDA<br />
OTHER TYPES OF DAMAGE TO CONIFERS .......................................................................................................................................................................................................................................... 124
a certain area or may occur over the entire<br />
tree but is generally restricted to the southern<br />
or southwestern exposure. <strong>Tree</strong>s with<br />
few branches are more likely to experience<br />
sunscald. Douglas-fi r is the most susceptible<br />
to sunscald, with true fi rs following closely<br />
behind (Fig. 5). Basal pruning in fall or<br />
winter and commercial tree wraps may help<br />
reduce this problem.<br />
Frost Injury<br />
Frost injury occurs in early spring and is most<br />
common on Douglas-fi r, true fi rs, and spruce.<br />
Pines are damaged only on rare occasions<br />
of extremely late season frost. <strong>Tree</strong>s that<br />
break bud early or are in frost-prone areas<br />
on northern slopes are most susceptible to<br />
this type of damage. Within a few days of the<br />
frost event, the new growth wilts and turns<br />
pinkish brown. As the season progresses,<br />
damaged shoots turn dark brown and may<br />
remain on the tree into summer (Fig. 6).<br />
Damaged growth can be removed during<br />
shearing, and the tree should recover within<br />
one year. Repeated frost damage will result<br />
in trees that are underdeveloped and bushy.<br />
Pines are best suited <strong>for</strong> planting in known<br />
frost pockets or on northern exposures.<br />
Figure 5. Douglas-fi r showing symptoms of<br />
sunscald. Courtesy of USDA Forest Service<br />
Ogden Archive, Bugwood.org (#1467233)<br />
Figure 6. New<br />
growth of Fraser<br />
fi r damaged by<br />
frost. Courtesy of<br />
Ricky Bates, Penn<br />
State<br />
Winter Injury<br />
Winter injury is more common with Fraser<br />
and Concolor fi r, eastern white pine, and<br />
Norway spruce. Both winter burn and drying<br />
may occur, causing the needles to turn<br />
brown. Occasionally, an entire tree will<br />
be discolored above the snow line (Figs. 7<br />
and 8). Needle loss may occur, especially<br />
on the southern side. On eastern white<br />
pine, winter injury causes needles to droop<br />
downward. On Scotch pine, yellow spots are<br />
found on damaged needles.<br />
Wind Damage<br />
Wind damage often occurs on the northwest<br />
or west side of the trees. It is also more<br />
common around the perimeter of the tree<br />
block. Needles will show discoloration from<br />
the tips of the needles to the back (Fig. 9).<br />
On closer inspection, needles may have a<br />
stippled effect.<br />
Figure 7. Snow<br />
protecting foliage<br />
from extreme<br />
winter elements.<br />
Courtesy of<br />
PDA<br />
Figure 8.<br />
Foliage that<br />
sat above<br />
the snow<br />
line exhibiting<br />
dieback.<br />
Courtesy of<br />
Rayanne D.<br />
Lehman, PDA<br />
Delayed Winter Dormancy<br />
Delayed winter dormancy may occur when<br />
climate conditions prior to and at the time<br />
of harvesting <strong>Christmas</strong> trees are warmer<br />
and/or wetter than usual. Typically, growers<br />
in the East begin harvesting trees in mid- to<br />
late November. When November temperatures<br />
are warmer than usual, trees may not<br />
have the opportunity to become dormant<br />
be<strong>for</strong>e being cut. When this occurs, trees are<br />
more likely to dry out and prematurely drop<br />
needles. Delaying tree cutting until after<br />
temperatures have dropped may be a way to<br />
prevent early needle loss.<br />
Figure 9. <strong>Tree</strong> near the perimeter<br />
of a block exhibiting wind<br />
damage. Courtesy of Rayanne D.<br />
Lehman, PDA<br />
ENVIRONMENTAL DAMAGE ................................................................................................................................................................................................................................................................ 125
Figure 1. Exhaust burn at<br />
the bottom of a tree.<br />
Courtesy of PDA<br />
Mechanical Damage<br />
Growing <strong>Christmas</strong> trees requires the<br />
use of many different types of equipment<br />
and tools. If not properly used, these may<br />
actually damage the trees.<br />
Equipment Damage<br />
Equipment used in a new tree block generally<br />
passes trouble free down the straight<br />
rows. As the trees grow, causing the rows to<br />
narrow, it becomes harder <strong>for</strong> the equipment<br />
to travel down the rows without causing<br />
damage. Equipment damage results from the<br />
tractor and its tires and implements.<br />
Equipment used <strong>for</strong> mowing and spraying<br />
can also cause damage to the trees.<br />
Entire small trees can be crushed and lower<br />
branches of larger trees can be broken or<br />
pulled from the trunk by equipment tires.<br />
The spray boom can scrape branches as<br />
well as damage bark and buds. Mowers are<br />
known to scrape the base of trees. <strong>Tree</strong>s at<br />
the ends of the rows are more susceptible to<br />
damage because of turning-radius requirements<br />
by the tractor and implements. It<br />
may take several weeks after the damage<br />
occurs <strong>for</strong> symptoms to appear, but they are<br />
most quickly noticeable around bud break.<br />
Equipment damage is often permanent and<br />
disfi guring.<br />
Some growers have modifi ed their equipment<br />
by adding guards to prevent branches<br />
of trees from being pulled into the path of<br />
the tires or implements. Damage can also be<br />
reduced by widening the distance between<br />
rows. Where the current standard is 5 feet<br />
by 5 feet (1.52 by 1.52 m), some farms are<br />
going to 5 feet by 6 feet (1.52 by 1.83 m) or<br />
even 6 feet by 6 feet (1.83 by 1.83 m). It is<br />
important to provide ample turning area at<br />
the end of rows when planting.<br />
Heat and fumes from equipment exhaust<br />
can damage trees. Exposure time does not<br />
have to be long, especially with new growth.<br />
Symptoms include discolored or browned<br />
needles and dying shoots (Fig. 1). The<br />
damaged area is generally about one square<br />
foot. Exhaust damage can be diagnosed by<br />
looking down the rows. If exhaust damage is<br />
likely, the burned spots will be at the same<br />
height on numerous trees in the row. Narrow<br />
rows are most susceptible.<br />
A few solutions <strong>for</strong> reducing this type of<br />
damage include directing exhaust away from<br />
trees, keeping hot engine parts away from<br />
trees, using a catalytic converter, and keeping<br />
equipment speeds increased rather than<br />
decreased.<br />
String trimmers cause serious damage to<br />
trees by damaging the bark at the base of the<br />
tree. This is especially true when trees are<br />
larger and the operator has diffi culty seeing<br />
into the trunk. The cuts and gouges in the<br />
bark offer entrance sites <strong>for</strong> several insect<br />
pests and diseases. To prevent string trimmer<br />
damage, maintain good weed control in the<br />
rows and avoid use of string trimmers under<br />
trees.<br />
Planting equipment may also lead to tree<br />
injury. If the planting depth is not correct,<br />
the taproot may curve upward instead of<br />
straight down. The tree roots will continue<br />
to grow upward—a condition known as<br />
J-rooting—and the trees will become<br />
stunted and eventually die (Fig. 2). To avoid<br />
J-rooting, adjust mechanical planters <strong>for</strong><br />
each use with the proper planting depth required<br />
<strong>for</strong> the size of transplants being used.<br />
Figure 2. A J-rooted tree. Courtesy of PDA<br />
OTHER TYPES OF DAMAGE TO CONIFERS .......................................................................................................................................................................................................................................... 126
Shearing Damage<br />
Shearing can damage trees. If a shearing<br />
knife is not used properly or is not sharp,<br />
branches will not be cut cleanly and partial<br />
cuts or broken branches may result. This<br />
results in dead or “fl agged” branches. To<br />
reduce damage when using a shearing knife,<br />
always use a sharp blade and swing with<br />
enough <strong>for</strong>ce to ensure minimal uncut<br />
branch ends (Fig. 3).<br />
Improper machine shearing can also<br />
harm trees. If the branches move away from<br />
the moving teeth, they will be left with a<br />
“chewed off” look. The end result is yellow,<br />
unattractive, or dead branch ends. <strong>Tree</strong>s<br />
can be damaged by excess lubricating oil<br />
and will appear burned. Avoid using too<br />
much when lubricating the blades. When<br />
machine shearing, keeping the equipment<br />
at the proper angle and speed while cutting<br />
into the branches will reduce the “chewed<br />
off” look of branch ends.<br />
Shearing is dangerous, so use all recommended<br />
safety equipment and provide<br />
training to those working on this activity.<br />
Figure 3. Clean, sharpened, 16-inch shearing<br />
knife. Courtesy of Sarah Pickel, PDA<br />
MECHANICAL DAMAGE ....................................................................................................................................................................................................................................................................... 127
Figure 1. Bird perching on a<br />
tree, which can cause the<br />
leaders to bend or break.<br />
Courtesy of Rayanne D.<br />
Lehman, PDA<br />
Vertebrate <strong>Pest</strong>s<br />
Birds<br />
Birds tend not to be a big problem associated<br />
with <strong>Christmas</strong> tree production, but damage<br />
occasionally occurs to the tops, particularly<br />
of taller trees (Fig. 1). This is especially common<br />
during spring, when the new growth<br />
is supple and not hardened off. The weight<br />
of the birds causes the terminal to bend and<br />
break. This damage is sometimes mistaken<br />
<strong>for</strong> white pine weevil damage; however, the<br />
birds will cause a sharp bend or break instead<br />
of the curled, wilted damage caused by the<br />
weevil. Taller trees are targeted because birds<br />
need a good view in order to defend their<br />
territory. Most growers having repeated damage<br />
will install poles or perches above tree<br />
height to alleviate the problem.<br />
Later in the year, birds may cause<br />
another type of damage as they perch on<br />
the stiff, mature terminals. Needles may be<br />
pulled from the terminal by the birds’ feet.<br />
This damage is minimal and will be corrected<br />
with another year’s growth. Noiseproducing<br />
devices have been used, but birds<br />
learn to tolerate them over time.<br />
Deer<br />
Deer will feed on all conifers, but spruce<br />
is the least preferred. Feeding damage may<br />
occur during winter when preferred foods<br />
are not available. The ends of deer-browsed<br />
shoots appear ragged because deer lack upper<br />
incisors and must bite down and rip food<br />
sources in order to feed (Fig. 2).<br />
Figure 2. Damage from deer browsing.<br />
Courtesy of John H. Ghent, USDA Forest<br />
Service, Bugwood.org (#0796079)<br />
The most common damage from deer<br />
occurs in late summer and fall when bucks<br />
polish their antlers by rubbing them against<br />
the trunks of small trees (Fig. 3). Damage<br />
may be more evident along the edge of<br />
blocks adjacent to woodlots as deer are an<br />
edge species. White-tailed deer cause more<br />
damage to <strong>Christmas</strong> trees than any other<br />
animal.<br />
Methods of limiting deer damage to<br />
<strong>Christmas</strong> trees include fencing and repellents.<br />
Fencing must be justifi able by being<br />
economical and of low maintenance. Studies<br />
on types of deer fence have shown three<br />
kinds to be effective. Woven wire fence is<br />
the best choice where deer damage is intolerable.<br />
Multistrand, electric, high-tensile<br />
fences are good in situations when total<br />
deer exclusion is not a necessity and the<br />
cost of fencing is not an issue. An electric<br />
poly-fence is good <strong>for</strong> seasonal protection.<br />
Each grower must look at all fencing options,<br />
farm operations, fi nances, and goals to<br />
decide the best choice <strong>for</strong> their farm.<br />
A second possible option <strong>for</strong> limiting<br />
deer damage is the use of repellents. Feed<br />
treated with lime provided the best repellency<br />
in one study; charcoal was the second<br />
most effective. Bittering agents were shown<br />
not to be effective over extended periods in<br />
another study. Growers have hung bars of<br />
Ivory soap from the trees with mixed results.<br />
Some other options <strong>for</strong> deterring deer<br />
are keeping a dog in the vicinity of the tree<br />
fi elds and allowing hunting on or near the<br />
property.<br />
Figure 3. Conifer bark and branches<br />
damaged by bucks polishing their antlers.<br />
Courtesy of Sarah Pickel, PDA<br />
OTHER TYPES OF DAMAGE TO CONIFERS .......................................................................................................................................................................................................................................... 128
Mice and Voles<br />
Damage to trees from these rodents is highest<br />
in the winter and early spring when a<br />
shortage of their preferred foods is present.<br />
During winter, both mice and voles will feed<br />
under the snow cover and consume bark<br />
cambium and phloem layers and the roots<br />
of trees (Fig. 4). A decrease in tree growth<br />
results from the sublethal feeding injuries,<br />
but smaller trees may die if completely<br />
girdled (Fig. 5).<br />
The best way to control these rodents<br />
is by maintaining weed control in the rows<br />
and particularly around the base of the tree.<br />
The bare ground is not attractive to the<br />
rodents and exposes them to predators.<br />
Figure 4. Rodent feeding on root tissue.<br />
Courtesy of PDA<br />
Figure 5. Girdling at the base of the tree<br />
from rodent feeding. Courtesy of Rayanne D.<br />
Lehman, PDA<br />
Rabbits<br />
Rabbits will feed on young trees. Shoots<br />
cut off at a 45-degree angle or girdling at<br />
the base are symptoms of rabbit feeding.<br />
Damage frequently occurs in winter when<br />
rabbits are unable to reach their normal<br />
food sources because of snow cover. During<br />
these periods, they will feed higher on the<br />
tree and may remove signifi cant amounts<br />
of bark. Good weed control in the rows and<br />
around the trees removes hiding places <strong>for</strong><br />
rabbits and makes them more vulnerable to<br />
predation.<br />
Groundhogs<br />
Groundhogs can do minimal chewing damage<br />
to tree bark, but the bigger problem is<br />
their burrows. These holes are hazardous<br />
to farm equipment and employees on foot.<br />
When groundhogs burrow under a tree,<br />
the root system is disturbed and the tree<br />
may be weakened. Maintaining good weed<br />
control, either chemically or by mowing,<br />
and eliminating brush piles will discourage<br />
groundhogs in the fi eld. Some recommendations<br />
include live trapping to relocate,<br />
smoke bombs, coyote or fox urine around<br />
the burrow, or motion devices to frighten<br />
these timid but destructive animals.<br />
VERTEBRATE PESTS .............................................................................................................................................................................................................................................................................. 129
PESTICIDE INFORMATION<br />
On most <strong>Christmas</strong> tree farms, pesticides play<br />
a role in management of pest issues. <strong>Pest</strong>icides<br />
are chemicals used to kill, mitigate, or repel<br />
pests; as such, both benefi ts and dangers may<br />
be associated with their use. To prevent misuse<br />
and undesirable effects, the government has<br />
enacted regulations on the use and handling of<br />
pesticides. This section discusses some of those<br />
pesticide regulations.<br />
In<strong>for</strong>mation on the safe use of pesticides<br />
can be found through county cooperative<br />
extension offi ces, state land-grant universities,<br />
state agricultural departments, or the United<br />
States Environmental Protection Agency. In<br />
Pennsylvania, Penn State’s <strong>Pest</strong>icide Education<br />
Program provides a wealth of educational<br />
materials. This in<strong>for</strong>mation can be found at<br />
www.pested.psu.edu.<br />
PESTICIDE INFORMATION .................................................................................................................................................................................................................................................................... 130
Fungicides, Herbicides,<br />
Insecticides, and Miticides<br />
<strong>Pest</strong>icides, by defi nition, are chemicals or<br />
other agents used to kill or control pests or<br />
to protect something from a pest. Generally,<br />
four types of pesticides are used in the <strong>Christmas</strong><br />
tree industry: fungicides used to control<br />
disease-causing fungi; herbicides to kill or<br />
deter plant growth; insecticides to control<br />
or prevent damage caused by insects; and<br />
miticides, often associated with insecticides,<br />
to control mites or arachnids (spiders and<br />
ticks). Each pesticide can be identifi ed by its<br />
brand name, active ingredient, and chemical<br />
class. Closely related chemicals are grouped<br />
in the same chemical class.<br />
<strong>Pest</strong>icide Resistance Issues<br />
One major concern that has been raised as<br />
the agricultural industry becomes increasingly<br />
dependent on pesticides is the risk of<br />
pests, including diseases and weeds, developing<br />
resistance to current pesticides. Repeated<br />
use of the same class of pesticide hastens this<br />
development and, ultimately, renders the<br />
chemical ineffective. This is especially true<br />
<strong>for</strong> pests that have multiple generations each<br />
growing season. By regularly changing to a<br />
compound from a different chemical class,<br />
the development of resistance is slowed, thus<br />
extending the useful life of the pesticide.<br />
Several international groups have<br />
<strong>for</strong>med to address the issue of pesticide<br />
resistance:<br />
• The Fungicide Resistance Action Committee<br />
(FRAC) has developed a number<br />
and letter code to distinguish the fungicide<br />
groups according to their cross-resistance<br />
behavior. A table of codes can be found at<br />
www.frac.info/frac/publication/anhang/<br />
FRAC_CODE_LIST.pdf.<br />
• The Herbicide Resistance Action<br />
Committee (HRAC) supports the global<br />
survey of resistant weeds initiated by the<br />
Weed Science Society of America. A<br />
searchable database of resistant weeds<br />
can be found at www.weedscience.com.<br />
• The Insecticide Resistance Action Committee<br />
(IRAC) has developed a mode<br />
of action (MOA) classifi cation based on<br />
known ways in which different products<br />
act. The MOA Classifi cation list can be<br />
downloaded from www.irac-online.org/<br />
News.asp.<br />
<strong>Pest</strong>icide Safety<br />
Federal and Pennsylvania <strong>Pest</strong>icide Laws<br />
The use of pesticides worldwide has increased<br />
greatly over the last 35 years.<br />
With this increased use, there are growing<br />
concerns <strong>for</strong> possible harm to human<br />
health and the environment. The purpose<br />
behind pesticide regulation is to protect<br />
public health and welfare, as well as prevent<br />
harmful effects on the environment through<br />
proper labeling, sale and distribution, transportation,<br />
storage, use and application, and<br />
disposal of pesticides. <strong>Pest</strong>icides are under<br />
regulatory inspection from the time they<br />
are invented in the lab to use in the fi eld or<br />
approved disposal.<br />
Since signed into law in 1947, the Federal<br />
Insecticide, Fungicide, and Rodenticide Act<br />
(FIFRA) has been the basis <strong>for</strong> regulation,<br />
distribution, and use of pesticides in the<br />
United States. FIFRA grants the federal<br />
Environmental Protection Agency the power<br />
to stop the sale or use of any pesticide. FIFRA<br />
regulations dictate labeling and packaging<br />
standards and also disposal procedures <strong>for</strong><br />
pesticide containers and surplus pesticides.<br />
The Pennsylvania <strong>Pest</strong>icide Control Act<br />
was enacted in 1973 as a companion bill to<br />
FIFRA. The purpose of this state act is to<br />
regulate the labeling, distribution, storage,<br />
transportation, use, application, and<br />
disposal of pesticides. In addition to being<br />
registered with EPA, every pesticide used in<br />
Pennsylvania must also be registered with<br />
the Pennsylvania Department of Agriculture.<br />
Regulations also cover dealers and<br />
applicators and the licensing of applicators.<br />
For additional in<strong>for</strong>mation, contact the<br />
Pennsylvania Department of Agriculture<br />
at www.paplants.state.pa.us/Index.aspx or<br />
Penn State’s <strong>Pest</strong>icide Education Program at<br />
www.pested.psu.edu. If you reside outside<br />
Pennsylvania, consult your local regulatory<br />
authority or land-grant university.<br />
Worker Protection Standard<br />
In August 1992, the Worker Protection<br />
Standard (WPS) was revised. Like FIFRA,<br />
it is overseen by the U.S. Environmental<br />
Protection Agency. This regulation is aimed<br />
at decreasing the risk of pesticide poisoning<br />
and injuries to agricultural workers and<br />
pesticide handlers. Included in the WPS are<br />
requirements <strong>for</strong> pesticide safety training,<br />
worker notifi cation of pesticide applications<br />
in the workplace, personal protective equipment<br />
use, reentry interval, decontamination<br />
supplies, and emergency medical assistance.<br />
CAUTION:<br />
Always read the<br />
pesticide label to<br />
determine specifi c<br />
uses and rates be<strong>for</strong>e<br />
mixing and applying<br />
the compound. If<br />
any questions arise,<br />
contact the dealer or<br />
manufacturer. It is<br />
illegal to apply any<br />
pesticide in excess of<br />
labeled rates. Labeled<br />
uses may vary <strong>for</strong><br />
each <strong>for</strong>mulation of<br />
the same chemical.<br />
Purchase the<br />
<strong>for</strong>mulation intended<br />
<strong>for</strong> your particular use.<br />
PESTICIDE INFORMATION .................................................................................................................................................................................................................................................................... 131
APPENDIXES
APPENDIX A<br />
PEST AND DISEASE<br />
PHOTO CHART<br />
This photographic appendix is designed to<br />
ease identifi cation of disease and insect pest<br />
problems. Two photographs are provided <strong>for</strong><br />
each pest, which are grouped according to<br />
the type of damage they represent. The fi rst<br />
photograph is an overall view of the damage<br />
and/or symptoms, while the second photograph<br />
is a close-up of the pest problem or<br />
the actual pest causing the symptoms. The<br />
fi rst column, “Name of <strong>Pest</strong>,” states what<br />
the problem is. The second column, “<strong>Tree</strong>s<br />
Susceptible,” indicates which trees are hosts<br />
<strong>for</strong> the particular pest. The page number of<br />
the appropriate pest fact sheet is provided<br />
in the third column.<br />
APPENDIX A ...................................................................................................................................................................................................................................................................................................................... APPENDIX A .......................................................................................................................................................................................................................................................................................... 134 134
<strong>Pest</strong> and Disease Photo Chart<br />
Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page<br />
Bagworm Douglas-fi r, fi r, pine, spruce 23 Cyclaneusma needle cast Pine 34<br />
S. GARDOSIK, PDA<br />
Balsam twig aphid Fir 26<br />
T. OLSON, PDA<br />
Cooley spruce gall adelgid Douglas-fi r 29<br />
(on Douglas-fi r)<br />
S. GARDOSIK, PDA<br />
Cryptomeria scale Douglas-fi r, fi r, pine, spruce 31<br />
S. GARDOSIK, PDA<br />
C. THOMAS, PDA<br />
R. LEHMAN, PDA<br />
S. GARDOSIK, PDA<br />
S. GARDOSIK, PDA<br />
NEEDLE DISCOLORATION AND INJURY<br />
USDA FOREST SERVICE<br />
Douglas-fi r needle midge Douglas-fi r 37<br />
Elongate hemlock scale Douglas-fi r, fi r, pine, spruce 39<br />
PEST AND DISEASE PHOTO CHART ................................................................................................................................................................................................................................................................................. 135<br />
T. OLSON, PDA<br />
S. GARDOSIK, PDA S. GARDOSIK, PDA<br />
T. OLSON, PDA<br />
S. GARDOSIK, PDA
Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page<br />
Eriophyid rust and sheath mites Pine, fi r, spruce 42 Introduced pine sawfl y Pine 53<br />
S. GARDOSIK, PDA<br />
European pine sawfl y Pine 53<br />
S. GARDOSIK, PDA<br />
Gypsy moth Douglas-fi r, fi r, pine, spruce 45<br />
PDA<br />
S. GARDOSIK, PDA<br />
NEEDLE DISCOLORATION AND INJURY (continued)<br />
S. KATOVICH, USDA FOREST SERVICE<br />
B. SCHILDT, PDA<br />
J. H. GHENT, USDA FOREST SERVICE<br />
Lophodermium needle cast Pine 48<br />
E. L. BARNARD, FLORIDA DEPARTMENT OF AGRICULTURE AND<br />
CONSUMER SERVICES<br />
Pine needle scale Douglas-fi r, fi r, pine, spruce 51<br />
C. THOMAS, PDA<br />
S. GARDOSIK, PDA<br />
J. H. GHENT, USDA FOREST SERVICE<br />
Ploioderma needle cast Pine 56<br />
APPENDIX A ...................................................................................................................................................................................................................................................................................................................... 136<br />
T. OLSON, PDA<br />
T. OLSON, PDA<br />
T. OLSON, PDA
Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page<br />
Red-band (Dothistroma)<br />
needle blight Pine 58<br />
T. OLSON, PDA<br />
Redheaded pine sawfl y Pine 53<br />
S. GARDOSIK, PDA<br />
T. OLSON, PDA<br />
Rhabdocline needle cast Douglas-fi r 60<br />
T. OLSON, PDA T. OLSON, PDA<br />
Rhizosphaera needle cast Spruce 63<br />
T. OLSON, PDA<br />
USDA FOREST SERVICE<br />
S. GARDOSIK, PDA<br />
Spruce needle rust Spruce 65<br />
Spruce spider mite Douglas-fi r, fi r, pine, spruce 67<br />
Swiss needle cast Douglas-fi r 70<br />
PEST AND DISEASE PHOTO CHART ................................................................................................................................................................................................................................................................................. 137<br />
T. OLSON, PDA<br />
E. R. DAY, VIRGINIA TECH<br />
T. OLSON, PDA<br />
T. OLSON, PDA<br />
S. GARDOSIK, PDA<br />
T. OLSON, PDA
Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page<br />
Atropellis canker Pine 73 Cooley spruce gall adelgid<br />
(on Colorado blue spruce) Spruce 80<br />
T. OLSON, PDA<br />
Balsam woolly adelgid Fir 75<br />
USDA FOREST SERVICE<br />
Botrytis blight Douglas-fi r, fi r, pine, spruce 78<br />
T. OLSON, PDA<br />
T. OLSON, PDA<br />
R. LEHMAN, PDA<br />
T. OLSON, PDA<br />
SHOOT AND BRANCH INJURY<br />
C. THOMAS, PDA<br />
S. GARDOSIK, PDA<br />
Diplodia (Sphaeropsis) tip blight Douglas-fi r, pine, spruce 82<br />
Eastern pine weevil Douglas-fi r, pine, spruce 84<br />
R. LEHMAN, PDA<br />
APPENDIX A ...................................................................................................................................................................................................................................................................................................................... 138<br />
T. OLSON, PDA<br />
W. JOHNSON, USDA FOREST SERVICE<br />
T. OLSON, PDA
Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page<br />
Eastern spruce gall adelgid Spruce 86 Pine shoot beetle Pine 94<br />
S. GARDOSIK, PDA R. LEHMAN, PDA<br />
Gall rusts Pine 88<br />
T. OLSON, PDA T. OLSON, PDA<br />
Pales weevil Douglas-fi r, pine, spruce 90<br />
S. GARDOSIK, PDA<br />
Pine bark adelgid Pine 92<br />
R. LEHMAN, PDA<br />
B. SCHILDT, PDA<br />
R. LEHMAN, PDA<br />
R. LEHMAN, PDA<br />
Striped pine scale Pine 97<br />
B. SCHILDT, PDA<br />
R. LEHMAN, PDA<br />
R. LEHMAN, PDA<br />
White pine blister rust Pine 99<br />
White pine weevil Douglas-fi r, fi r, pine, spruce 101<br />
PEST AND DISEASE PHOTO CHART ................................................................................................................................................................................................................................................................................. 139<br />
T. OLSON, PDA<br />
PDA<br />
T. OLSON, PDA<br />
S. KATOVICH, USDA FOREST SERVICE
Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page<br />
Armillaria root rot Douglas-fi r, fi r, pine, spruce 107 Phytophthora root rot Fir, pine, spruce 111<br />
J. O’BRIEN, USDA FOREST SERVICE<br />
Bark and engraver beetles Pine 109<br />
PDA<br />
J. STIMMEL, PDA<br />
Japanese beetle Douglas-fi r, pine, spruce 118<br />
STEM AND ROOT INJURY/TREE MORTALITY<br />
D. POWELL, USDA FOREST SERVICE<br />
S. GARDOSIK, PDA<br />
S. GARDOSIK, PDA<br />
Pine root collar weevil Pine 113<br />
Pine wilt disease Pine 116<br />
APPENDIX A ...................................................................................................................................................................................................................................................................................................................... 140<br />
T. OLSON, PDA<br />
S. GARDOSIK, PDA<br />
A. S. MUNSON, USDA FOREST SERVICE<br />
T. OLSON, PDA<br />
J. B. HANSON, USDA FOREST SERVICE<br />
L. D. DWINELL, USDA FOREST SERVICE
Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page<br />
White grubs Douglas-fi r, fi r, pine, spruce 118 Zimmerman pine moth Pine, spruce 120<br />
Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page<br />
Air pollution<br />
(environmental damage) Douglas-fi r, fi r, pine, spruce 124<br />
MINNESOTA DEPARTMENT OF NATURAL RESOURCES<br />
S. KATOVICH, USDA FOREST SERVICE<br />
J. STIMMEL, PDA<br />
OTHER TYPES OF DAMAGE TO CONIFERS<br />
MINNESOTA DEPARTMENT OF NATURAL RESOURCES<br />
W. CRANSHAW, COLORADO STATE UNIVERSITY<br />
W. CRANSHAW, COLORADO STATE UNIVERSITY<br />
Drought<br />
(environmental damage) Douglas-fi r, fi r, pine, spruce 124<br />
Chemical damage Douglas-fi r, fi r, pine, spruce 123 Frost injury<br />
(environmental damage) Douglas-fi r, fi r, pine, spruce 125<br />
R. BATES, PENN STATE<br />
T. OLSON, PDA<br />
W. M. BROWN JR.<br />
R. BATES, PENN STATE<br />
R. L. ANDERSON, USDA FOREST SERVICE<br />
R. BATES, PENN STATE<br />
PEST AND DISEASE PHOTO CHART ................................................................................................................................................................................................................................................................................. 141
Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page Name of <strong>Pest</strong> <strong>Tree</strong>s Susceptible Page<br />
Heat (environmental damage) Douglas-fi r, fi r, pine, spruce 124 Vertebrate pests Douglas-fi r, fi r, pine, spruce 128<br />
R. BATES, PENN STATE<br />
Mechanical damage Douglas-fi r, fi r, pine, spruce 126<br />
PDA<br />
Sunscald<br />
(environmental damage) Douglas-fi r, fi r 124<br />
USDA FOREST SERVICE OGDEN<br />
PDA<br />
C. THOMAS, PDA<br />
S. K. HAGLE, USDA FOREST SERVICE<br />
OTHER TYPES OF DAMAGE TO CONIFERS (continued)<br />
USDA FOREST SERVICE NORTH CENTRAL RESEARCH STATION<br />
Winter injury<br />
(environmental damage) Douglas-fi r, fi r, pine, spruce 125<br />
R. LEHMAN, PDA<br />
R. LEHMAN, PDA<br />
E. G. VALLERY, USDA FOREST SERVICE<br />
APPENDIX A ...................................................................................................................................................................................................................................................................................................................... 142
APPENDIX B<br />
BIOLOGICAL CONTROLS<br />
PHOTO CHART<br />
This photographic appendix is designed<br />
to help with identifying biological<br />
controls, or biocontrols (both insects<br />
and fungi). The fi rst column, “Targeted<br />
<strong>Pest</strong>(s),” indicates pest(s) on which<br />
each biocontrol will prey. The common<br />
name and a photograph of each biocontrol<br />
appear in the second column,<br />
which is arranged alphabetically by<br />
biocontrol common name. The third<br />
column provides the scientifi c name<br />
of the biocontrol being viewed.<br />
APPENDIX B APPENDIX ...................................................................................................................................................................................................................................................................................................................... B .......................................................................................................................................................................................................................................................................................... 144 144
Targeted <strong>Pest</strong>(s) Biological Control Scientifi c Name<br />
Cryptomeria scale Aphytis (parasitoid) wasp Aphytis spp.<br />
Aphids (balsam<br />
twig, etc.); adelgids<br />
(balsam woolly, pine<br />
bark, Cooley spruce<br />
gall); other small,<br />
soft-bodied insects<br />
Wood borers, woodinhabiting<br />
beetles<br />
S. PICKEL, PDA<br />
Biological Controls Photo Chart<br />
Brown lacewing Hemerobius stigma<br />
Stephens<br />
R. LEHMAN, PDA<br />
Checkered beetle Cleridae family<br />
R. LEHMAN, PDA<br />
Scales Cn beetle (predatory beetle) Cybocephalus<br />
nipponicus<br />
Endrödy-Younga<br />
B. SCHILDT, PDA<br />
Targeted <strong>Pest</strong>(s) Biological Control Scientifi c Name<br />
Aphids, beetle larvae,<br />
caterpillars (small),<br />
moths, spider mites<br />
Douglas-fi r needle<br />
midge<br />
Common green lacewing Chrysoperla<br />
rufi labris<br />
(Burmeister)<br />
Crab spider Thomisidae family<br />
Mite eggs and adults Dusty wing Coniopterygidae<br />
family<br />
Adelgids, aphids,<br />
adult beetles, beetle<br />
larvae, caterpillars,<br />
fl ies, mites, other pest<br />
insects<br />
B. SCHILDT, PDA<br />
R. LEHMAN, PDA<br />
C. MOOREHEAD<br />
Entomopathogenic fungi Isaria sp.<br />
M. BARBERCHECK, PENN STATE<br />
Aphids Eye-spotted lady beetle Anatis mali (Say)<br />
R. LEHMAN, PDA<br />
BIOLOGICAL CONTROLS PHOTO CHART ......................................................................................................................................................................................................................................................................... 145
Targeted <strong>Pest</strong>(s) Biological Control Scientifi c Name<br />
Aphids Fifteen-spotted lady beetle Anatis labiculata<br />
(Say)<br />
Aphids Golden-eyed lacewing Chrysopa oculata<br />
(Say)<br />
Aphids Hudsonian lady beetle Mulsantina<br />
hudsonica (Casey)<br />
Aphids, insect eggs,<br />
misc. larvae/nymphs,<br />
mites<br />
A. BATEMAN, BUGGUIDE.NET<br />
CLEMSON UNIVERSITY, USDA COOPERATIVE EXTENSION<br />
SLIDE SERIES<br />
J. BAILEY, BUGGUIDE.NET<br />
Insidious fl ower bug, minute pirate<br />
bug<br />
W. CRANSHAW, COLORADO STATE UNIVERSITY<br />
Orius spp.<br />
Armored scales Lady beetle Microweisia spp.<br />
R. COWLES, CONNECTICUT AGRICULTURAL EXPERIMENT<br />
STATION<br />
Scales Lady beetle (white ladybug larva) Hyperaspis spp.<br />
R. LEHMAN, PDA<br />
Targeted <strong>Pest</strong>(s) Biological Control Scientifi c Name<br />
Adelgids (exposed<br />
stages), aphids, mites<br />
Lady beetle Stethorus spp.<br />
Scales Lindorus beetle Lindorus<br />
lophanthae<br />
(Blaisd.)<br />
Various insects Mantids Mantidae family<br />
Aphids, scale insects,<br />
other soft-bodied<br />
insects<br />
F. PEAIRS, COLORADO STATE UNIVERSITY<br />
RINCON-VITOVA INSECTARIES<br />
C. THOMAS, PDA<br />
Multicolored Asian lady beetle Harmonia axyridis<br />
(Pallas)<br />
PENNSYLVANIA DCNR<br />
Armored scales Parasitic wasp Encarsia citrina<br />
(Craw<strong>for</strong>d)<br />
R. COWLES, CONNECTICUT AGRICULTURAL EXPERIMENT<br />
STATION<br />
Aphids Pine lady beetle Mulsantina picta<br />
(Randall)<br />
C. MOOREHEAD<br />
APPENDIX B ...................................................................................................................................................................................................................................................................................................................... 146
Targeted <strong>Pest</strong>(s) Biological Control Scientifi c Name<br />
Caterpillars, sawfl y<br />
larvae<br />
Potter wasp Eumenes fraternus<br />
Say<br />
Spider mites Predatory mite Neoseiulus fallacis<br />
(Garman)<br />
Hemlock woolly<br />
adelgid<br />
Caterpillars, sawfl y<br />
larvae, other insects<br />
Caterpillars, sawfl y<br />
larvae<br />
R. BROEKHUIS, BUGGUIDE.NET<br />
NORTH CAROLINA STATE UNIVERSITY<br />
Pt beetle (lady beetle) Pseudoscymnus<br />
tsugae Sasajii and<br />
McClure<br />
D. J. SOUTO, USDA FOREST SERVICE<br />
Shield/stink bug Pentatomidae<br />
family<br />
R. L. ANDERSON, USDA FOREST SERVICE<br />
Sphecid wasp Ammophila spp.<br />
J. LAWRENCE, EUROFINS AGROSCIENCE SERVICES<br />
Targeted <strong>Pest</strong>(s) Biological Control Scientifi c Name<br />
Aphids, other softbodied<br />
insects<br />
Syrphid fl y (hover fl y) Syrphidae family<br />
Aphids Three-banded lady beetle Coccinella<br />
trifasciata<br />
Linnaeus<br />
Aphids Transverse lady beetle Coccinella<br />
transversoguttata<br />
Falderman<br />
Aphids, scales Twice-stabbed lady beetle Chilocorus orbus<br />
Casey<br />
Immature and adult<br />
insects<br />
C. THOMAS, PDA<br />
T. MURRAY, BUGGUIDE.NET<br />
W. CRANSHAW, COLORADO STATE UNIVERSITY<br />
R. LEHMAN, PDA<br />
Yellow jacket/wasp Vespula spp.<br />
R. LEHMAN, PDA<br />
BIOLOGICAL CONTROLS PHOTO CHART ......................................................................................................................................................................................................................................................................... 147
APPENDIX C<br />
CONIFER SPECIES<br />
These conifer species are mentioned as<br />
pest hosts in this manual or are common<br />
tree species grown in the Northeast.<br />
Conifers sold as <strong>Christmas</strong> trees are<br />
highlighted.<br />
APPENDIX C .......................................................................................................................................................................................................................................................................................... 148
Conifer Species<br />
Genus (Group) Common Name Species Name<br />
Abies (true fi rs) Balsam fi r Abies balsamea (L.) Mill<br />
Canaan fi r Abies balsamea var. phanerolepis (Fern.)<br />
Concolor fi r or white fi r Abies concolor (Gord. and Glend.) Lindl.<br />
Fraser fi r Abies fraseri (Pursh) Poir.<br />
Grand fi r Abies grandis (Douglas ex D. Don) Lindl.<br />
Korean fi r Abies koreana (Sieb and Zucc.)<br />
Momi fi r Abies fi rma (Sieb and Zucc.)<br />
Noble fi r Abies procera Rehder<br />
Nordmann fi r Abies nordmanniana (Steven) Spach<br />
Turkish fi r Abies bournmuelleriana (Mattf.)<br />
Juniperus (junipers) Eastern red cedar Juniperus virginiana L.<br />
Picea (spruce) Black hills spruce Picea glauca var. densata Bailey<br />
Colorado blue spruce Picea pungens Engelm.<br />
Englemann spruce Picea engelmannii Parry ex Engelm.<br />
Norway spruce Picea abies (L.) H. Karst.<br />
Serbian spruce Picea omorika (Pancic) Purk.<br />
Sitka spruce Picea sitchensis (Bong.) Carrière<br />
White spruce Picea glauca (Moench) Voss<br />
Pinus (pines) Austrian pine Pinus nigra J. F. Arnold<br />
Eastern white pine Pinus strobus L.<br />
Jack pine Pinus banksiana Lamb.<br />
Lodgepole pine Pinus contorta Douglas ex Louden<br />
Monterey pine Pinus radiata D. Don<br />
Mugo pine Pinus mugo Turra<br />
Ponderosa pine Pinus ponderosa P. Lawson and C. Lawson<br />
Red pine Pinus resinosa Aiton<br />
Scotch pine Pinus sylvestris L.<br />
Virginia pine Pinus virginiana (Mill.)<br />
Western white pine Pinus monticola (Douglas ex D. Don)<br />
Pseudotsuga (Douglas-fi r) Douglas-fi r Pseudotsuga menziesii (Mirb.) Franco<br />
Thuja (cedar) Arborvitae or northern white cedar Thuja occidentalis L.<br />
Tsuga (hemlock) Eastern hemlock Tsuga canadensis (L.) Carrière<br />
Conifers sold as <strong>Christmas</strong> trees are highlighted.<br />
CONIFER SPECIES .................................................................................................................................................................................................................................................................................. 149
APPENDIX D<br />
SEASONAL MONITORING GUIDE<br />
A good scouting program <strong>for</strong> your <strong>Christmas</strong> tree<br />
farm can lead to a more accurate pest and disease<br />
control program. Set time aside to scout <strong>for</strong> new<br />
insect and disease problems several times a year.<br />
Also take time to intensively scout <strong>for</strong> known<br />
pests to best determine the need <strong>for</strong> and timing<br />
of controls. As a rule, every time you are in<br />
a block of trees <strong>for</strong> planting, spraying, shearing,<br />
mowing, or harvesting, make note of any insect<br />
or disease issues. Combine these scouting tips<br />
with the control recommendations in the corresponding<br />
pest fact sheets found in this manual.<br />
Be prepared with the proper scouting tools<br />
be<strong>for</strong>e beginning these scouting activities. The<br />
fi rst and most important tool is a hand lens of<br />
15X magnifi cation or higher. Other tools include<br />
pruners to clip branches <strong>for</strong> sampling, plastic<br />
bags or vials to collect samples, and a pencil or<br />
pen and paper to record your fi ndings.<br />
Disclaimer: The suggested timing of the following<br />
scouting recommendations are based on<br />
general pest activity times in the Mid-Atlantic<br />
region. The best way to adapt this calendar to<br />
your region of the country is to follow the growing<br />
degree day timings listed in the fact sheet<br />
section of this manual. Also, the specifi c tree<br />
varieties on your farm may require that your<br />
scouting activities include pests not listed in<br />
this section.<br />
APPENDIX D .......................................................................................................................................................................................................................................................................................... 150
Seasonal Monitoring Guide<br />
Time of Year Hosts <strong>Pest</strong> How to Scout<br />
Late winter to early<br />
spring (March to<br />
early April), be<strong>for</strong>e<br />
bud break<br />
True fi rs Balsam twig aphid • Look <strong>for</strong> trees with curled last season’s needles.<br />
• On undamaged shoot, look <strong>for</strong> silvery,<br />
oval-shaped eggs on underside of twig within<br />
1–2 inches of buds.<br />
• Light green stem mothers feed on undersides of<br />
needles, excreting honeydew droplets.<br />
Colorado blue spruce Cooley spruce gall adelgid • Look <strong>for</strong> brown, pinecone-like galls from the<br />
previous season.<br />
• Gray-black overwintering nymphs are found at<br />
the base of the new season’s buds.<br />
Douglas-fi r Cooley spruce gall adelgid • Look <strong>for</strong> crooked needles.<br />
• Black nymphs will be on undersides of needles<br />
(may be covering over with white wax).<br />
Norway spruce Eastern spruce gall adelgid • Look <strong>for</strong> brown galls at the base of previous<br />
year’s growth; needles may be missing beyond<br />
gall.<br />
• The black overwintering nymphs will be found<br />
at the base of the new season’s buds.<br />
Pines, spruces, true fi rs Eriophyid mites • Look at 20 trees per acre that exhibit a gray or<br />
rusty coloring.<br />
• Tiny, peach-colored eggs are found in clusters or<br />
rows at the needle base (underside).<br />
• Pale, oblong adult mites move along the<br />
needles.<br />
Pines Pales weevil • Look <strong>for</strong> trees showing “fl agging,” or browning,<br />
of needles at the ends of branches that<br />
have shown signs of chewing on stem bark.<br />
• Pull duff away from base of last year’s stumps to<br />
look <strong>for</strong> adult weevils that are becoming active.<br />
Douglas-fi r Rhabdocline needle cast • Scout trees in areas likely to hold extra moisture<br />
such as shaded areas along a woodlot or tight<br />
plantings.<br />
• Examine current season’s needles <strong>for</strong> orangerust<br />
discoloration.<br />
Spruces Rhizosphaera needle cast • On the lower half of the tree, look <strong>for</strong> purplebrown<br />
needles with visible black fruiting bodies.<br />
Spruces Spruce needle rust • On cloudy days, look <strong>for</strong> needles with yellow<br />
bands containing an orange spot.<br />
• Focus scouting in low or shaded areas.<br />
Arborvitae, Douglas-fi r,<br />
spruces, true fi rs<br />
Spruce spider mites • Look <strong>for</strong> discolored (brown or yellow) needles.<br />
• Red eggs are found on the undersides of twigs,<br />
needles, and at base of buds.<br />
• Reddish-brown adult mites move along needles<br />
and twigs.<br />
• Tap symptomatic branches over a white surface<br />
to dislodge eggs or mites.<br />
Douglas-fi r Swiss needle cast • In shady or tightly planted areas, look <strong>for</strong> trees<br />
with needles that are browning from the tip<br />
down.<br />
• On the underside of these needles, rows of tiny,<br />
black fruiting bodies will be visible with a hand<br />
lens.<br />
• Also look <strong>for</strong> fruiting bodies on healthy,<br />
green needles.<br />
(continued)<br />
SEASONAL MONITORING GUIDE ........................................................................................................................................................................................................................................................ 151
Seasonal Monitoring Guide (continued)<br />
Time of Year Hosts <strong>Pest</strong> How to Scout<br />
Late winter to early<br />
spring (March to<br />
early April), be<strong>for</strong>e<br />
bud break<br />
(continued)<br />
Midspring to early<br />
summer (early April<br />
to June)<br />
Harvest (November<br />
to December)<br />
Douglas-fi r, pines,<br />
spruces<br />
Arborvitae, Douglas-fi r,<br />
pines, spruces, true fi rs<br />
White pine weevil • Set out baited pyramidal traps (see Appendix E<br />
<strong>for</strong> instructions).<br />
• Check soil temperature daily on unshaded side<br />
of a tree in susceptible block.<br />
• Once soil temperatures near 50°F, check traps<br />
daily.<br />
• On sunny days, look <strong>for</strong> sap droplets on tree<br />
leaders as feeding evidence.<br />
Bagworm • Look <strong>for</strong> trees with brown, conelike bags,<br />
1–2 inches in length hanging from the previous<br />
season’s growth.<br />
• Near the end of May, begin to look <strong>for</strong> tiny<br />
larvae feeding on the new season’s needles.<br />
True fi rs Balsam woolly adelgid • Look at the tops of trees <strong>for</strong> crooked leaders.<br />
• On symptomatic trees, try to bend the main<br />
trunk. <strong>Tree</strong>s with balsam woolly adelgid will be<br />
extra stiff.<br />
• Branches will have gouty enlargements, or<br />
“warts.”<br />
• Small, purplish-black insects will be under the<br />
white, woolly wax on the bark.<br />
Douglas-fi r, spruces, true<br />
fi rs<br />
Cryptomeria scale • Lift the lowest branches of select trees to look<br />
<strong>for</strong> yellow speckling on top of needles.<br />
• Examine underside of needles <strong>for</strong> off-white,<br />
oval-shaped scales with yellow centers. Look<br />
closely with hand lens <strong>for</strong> moving, yellow,<br />
oblong crawlers among the scales.<br />
• In August, monitor <strong>for</strong> the second generation.<br />
Douglas-fi r Douglas-fi r needle midge • Prior to bud break, set out cardboard emergence<br />
traps (see Douglas-fi r needle midge<br />
fact sheet) at the base of trees with kinked or<br />
damaged needles from the previous season.<br />
• As buds swell, monitor traps <strong>for</strong> delicate, orange<br />
midges in the capture jar.<br />
• Emerging midges will also be found on breaking<br />
buds or hovering close by.<br />
Douglas-fi r, hemlocks,<br />
spruces, true fi rs<br />
Douglas-fi r, pines,<br />
spruces, true fi rs<br />
Elongate hemlock scale • Look <strong>for</strong> yellowing needles with a gray cast at<br />
the base of the trees, near the trunk.<br />
• Examine the underside of the needles <strong>for</strong><br />
mobile, yellow, oblong crawlers among the<br />
narrow, brown scales and shorter, white scales.<br />
• Tapping the branch over a white paper or<br />
clipboard may dislodge the crawlers.<br />
• Monitor <strong>for</strong> continuing generations throughout<br />
summer.<br />
Pine needle scale • White, oblong scales can be found on the most<br />
recent year’s needles.<br />
• Look underneath the white scales to see if the<br />
purplish-red eggs have begun to hatch.<br />
• Paprika-colored crawlers will be visible on the<br />
needles.<br />
• Look <strong>for</strong> second generation in July.<br />
True fi rs Balsam woolly adelgid • Observe cut stumps <strong>for</strong> indications of the red<br />
reactionary wood.<br />
Douglas-fi r, pines,<br />
spruces, true fi rs<br />
Douglas-fi r, hemlocks,<br />
spruces, true fi rs<br />
Cryptomeria scale • Observe underside of branches <strong>for</strong> presence of<br />
scale.<br />
Elongate hemlock scale • Observe underside of branches <strong>for</strong> presence of<br />
scale.<br />
APPENDIX D .......................................................................................................................................................................................................................................................................................... 152
APPENDIX E<br />
INSECT TRAP USE AND<br />
CONSTRUCTION<br />
The use of traps <strong>for</strong> insect detection<br />
is an important method in any IPM<br />
program. Traps can provide an accurate<br />
picture of pest presence on a farm and<br />
may also allow <strong>for</strong> proper timing of<br />
pesticide applications. This proper timing<br />
ensures that the pesticides used will<br />
be effective and may ultimately lead to<br />
a reduction in the amount of pesticides<br />
used. On the following pages are<br />
descriptions of three insect traps that<br />
can be easily used in <strong>Christmas</strong> tree<br />
plantations.<br />
APPENDIX E .......................................................................................................................................................................................................................................................................................... 154
White Pine Weevil<br />
Detection Traps<br />
(Adapted from Regulatory Horticulture<br />
article by Sandy Gardosik and Rayanne D.<br />
Lehman, PDA)<br />
The Tedder’s trap, a baited, pyramid-shaped<br />
trap (Fig. 1), is used <strong>for</strong> monitoring the<br />
spring emergence of adult white pine weevils.<br />
This insect feeds on the leaders of most<br />
conifer trees. The dark, pyramid-shaped<br />
base of the trap resembles the general shape<br />
of a conifer, the alcohol and turpentine<br />
baits mimic the odors emitted by wounded<br />
conifers, and the funnel top captures the<br />
weevils much like a minnow trap. Per<strong>for</strong>mance<br />
of these traps can be greatly enhanced<br />
by monitoring soil temperatures at<br />
the trap location. (For more details, see the<br />
White Pine Weevil fact sheet on page 101.)<br />
Ordering<br />
The Whalon Modifi ed Tedder’s trap is sold<br />
by Great Lakes IPM. Each trap kit includes<br />
a 4-foot-tall base, a trap top, and 2 stakes.<br />
This trap is also advertised as a plum curculio<br />
and pecan weevil trap. The version sold<br />
as a white pine weevil trap includes 2 plastic<br />
vials to be used <strong>for</strong> the alcohol and turpentine<br />
bait. This product is listed as catalog<br />
# IPM-502 and sells <strong>for</strong> $16.50 in the 2010<br />
catalog.<br />
The top assembly, which consists of a<br />
plastic funnel and cylinder (Fig. 2), can<br />
be purchased separately. It is listed as the<br />
Boll Weevil Top Assembly, catalog # IPM-<br />
1006T, and sells <strong>for</strong> $1.75 (2010 catalog).<br />
Contact Great Lakes IPM by phone at<br />
1-800 235-0285 or online at www.greatlakesipm.com.<br />
Figure 1.<br />
Pyramidal<br />
Tedder’s trap.<br />
Courtesy of<br />
Brian Schildt,<br />
PDA<br />
Building a Trap Base<br />
The trap base must be prepared if only<br />
the trap top is purchased or if a base has<br />
deteriorated with usage.<br />
• The base consists of two triangles (see Fig.<br />
3 <strong>for</strong> dimensions) that may be constructed<br />
from any outdoor hearty material (plywood,<br />
corrugated plastic, etc.). A material<br />
with a rougher surface will provide a better<br />
climbing surface <strong>for</strong> the weevils. Since<br />
the base is intended to resemble a tree<br />
(to the weevils), the material should be<br />
dark in color—dark brown, dark green, or<br />
black. Paint with sand added would also<br />
provide a good gripping surface <strong>for</strong> the<br />
weevils.<br />
• When cutting out the triangles, cut a<br />
slot 24 inches long in the bottom of one<br />
triangle, which will be vane A. A similar<br />
slot 24 inches long, vane B, must be cut in<br />
the top of the other triangle.<br />
After the triangles are<br />
cut, holes must be drilled<br />
in the top of vane “A”<br />
(solid top, slotted<br />
bottom) to attach the<br />
bait.<br />
In addition, holes must<br />
be cut in the bottom<br />
of each vane, near the<br />
exterior angles. These<br />
holes will be used to<br />
stake the trap in place in<br />
the plantation.<br />
Figure 3. Building the trap. Courtesy of Rayanne D. Lehman, PDA<br />
Figure 2.<br />
Weevil trap top<br />
assembly.<br />
Courtesy of<br />
Cathy Thomas,<br />
PDA<br />
INSECT TRAP USE AND CONSTRUCTION ............................................................................................................................................................................................................................................. 155
Figure 4. Bait vials attached to<br />
upper portion of the trap.<br />
Courtesy of Brian Schildt, PDA<br />
Figure 5. A kit containing baits,<br />
collection bags, and all other<br />
trap supplies. Courtesy of Cathy<br />
Thomas, PDA<br />
Figure 6. Securely wire top to<br />
the base. Courtesy of Cathy<br />
Thomas, PDA<br />
Figure 7. Weevils caught in trap<br />
(plastic vial has been removed).<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
Trap Assembly<br />
1. Slide triangle A (slot in bottom) over<br />
the top of triangle B (slot on top). The<br />
bottom and top edges of both triangles<br />
should be even. When assembled properly,<br />
the base will stand freely with four<br />
vanes.<br />
2. Using fi ne-gauge wire, utilize the small<br />
holes along the top of vane A to attach<br />
two small bottles. These may be the<br />
vials provided with the trap kit or other<br />
small bottles (Fig. 4). Inexpensive,<br />
small, glass salt and pepper shakers are a<br />
good option. The bottles should lie fl at<br />
against the angle created by the vanes<br />
and have their tops as close as possible<br />
to the top of the base.<br />
3. Fill one bottle with 95 percent ethyl<br />
alcohol (available in stores selling wine<br />
and spirits) or denatured alcohol (available<br />
in hardware stores); fi ll the other<br />
with gum turpentine (Fig. 5). Unless<br />
the bottle lids have holes, do not put<br />
lids on the bottles.<br />
4. Fit the trap top over the base, covering<br />
the tops of the bottles containing the<br />
alcohol and turpentine. Use fi ne-gauge<br />
wire or plastic twist ties to secure the<br />
top to the holes at the top of the trap<br />
base (Fig. 6).<br />
5. Twist the plastic vial over the funnel<br />
to screw into place. (The funnel and<br />
vial may come already assembled.) This<br />
is where the weevils will be trapped<br />
(Fig. 7) after crawling up the vane and<br />
entering through the small opening in<br />
the screening.<br />
Figure 8. Soil<br />
thermometer.<br />
Courtesy of Cathy<br />
Thomas, PDA<br />
Using the Traps<br />
1. Weevils become active when air temperatures<br />
exceed 50°F and soil temperatures<br />
reach 50°F (Fig. 8). Earliest<br />
emergence occurs in the warmest fi elds.<br />
For best results, the traps must be in<br />
place be<strong>for</strong>e the pests are active on a<br />
daily basis. Place the traps in the fi eld to<br />
be monitored in late winter (February)<br />
or early spring (early March).<br />
2. Locate each trap in the tree row, preferably<br />
next to a tree that has had weevil<br />
damage the previous season (Fig. 9).<br />
Prevent the trap from blowing over by<br />
using tent stakes, wire (if necessary),<br />
and the holes in bottoms of trap base.<br />
Do not use string or twine, as small<br />
animals will chew this and may destroy<br />
the base in the process.<br />
3. Weevils overwintering at the bases of<br />
trees will be attracted by the scents<br />
given off by the evaporation of the alcohol<br />
and turpentine. When they become<br />
active, they will climb the base of the<br />
trap and enter the funnel portion at the<br />
top. Once inside the funnel, they will<br />
remain inside the large plastic vial until<br />
removed.<br />
Figure 9. White pine weevil damage<br />
from previous season. Courtesy of<br />
Steven Katovich, USDA Forest Service,<br />
Bugwood.org (#5202043)<br />
APPENDIX E .......................................................................................................................................................................................................................................................................................... 156
4. Generally, two traps per block are<br />
suffi cient. For extremely large blocks,<br />
increase the number of traps.<br />
5. Replenish the contents of the alcohol<br />
and turpentine bottles regularly. If rain<br />
has occurred, the bottles should be<br />
emptied and fi lled with fresh material.<br />
Comparison trials in Pennsylvania<br />
showed that unbaited traps did not<br />
capture weevils.<br />
6. Check the traps <strong>for</strong> weevils daily, if possible,<br />
during early spring. If any insects<br />
are in the trap, empty the contents into<br />
a container of soapy water or rubbing<br />
alcohol to kill the pests.<br />
7. Apply a registered pesticide to the<br />
terminals of susceptible trees as soon as<br />
possible after the fi rst white pine weevil<br />
is captured. During cool springs, it may<br />
be necessary to repeat the application if<br />
weevils continue to appear in the traps<br />
4–6 weeks after the fi rst application.<br />
Follow all label directions <strong>for</strong> application<br />
of any pesticide.<br />
8. These traps are <strong>for</strong> detection of weevil<br />
activity and will not offer control.<br />
Identifying the Weevils<br />
Several weevil species, as well as spiders,<br />
fl ies, stonefl ies, and other beetles, will be<br />
attracted to the traps (Fig. 10). It is important<br />
<strong>for</strong> the grower to properly identify the<br />
weevils that are associated with <strong>Christmas</strong><br />
tree production. Four such species exist in<br />
Pennsylvania: white pine weevil, eastern<br />
(<strong>for</strong>merly northern) pine weevil, pales weevil,<br />
and pine root collar weevil. Both white<br />
pine weevil and eastern pine weevil are very<br />
similar in appearance. They both are dark<br />
red brown and have large, white and gold<br />
patches on the back of their wing coverings.<br />
To the untrained and unaided eye, the main<br />
difference is their size. The target species,<br />
white pine weevil, is the smaller of the two.<br />
Pales weevil and pine root collar weevil<br />
will also be attracted to the traps. They can<br />
easily be separated from the previous two<br />
species by size and color but are diffi cult to<br />
separate from each other. These weevils are<br />
larger and dark brown to almost black with<br />
small, white patches on the top of their<br />
wing coverings. Pine root collar weevil is<br />
the larger of these two species.<br />
Figure 10. Pales weevil (left),<br />
and white pine weevil (right).<br />
Courtesy of Gerald J. Lenhard,<br />
Louisiana State University,<br />
Bugwood.org (#0795087)<br />
INSECT TRAP USE AND CONSTRUCTION ............................................................................................................................................................................................................................................. 157
Figure 11. Box traps placed<br />
at the base of a previously<br />
infected tree. Courtesy of<br />
Sandy Gardosik, PDA<br />
Figure 12. Inexpensive home<br />
materials used <strong>for</strong> traps.<br />
Courtesy of Sandy Gardosik,<br />
PDA<br />
Figure 13. Jar secured with duct<br />
tape in the box. Courtesy of<br />
Sandy Gardosik, PDA<br />
Douglas-Fir Needle<br />
Midge Emergence Trap<br />
This home-constructed trap is a ground<br />
trap used <strong>for</strong> monitoring Douglas-fi r needle<br />
midge (Fig. 11). This insect emerges in<br />
spring as an adult and lays eggs in the<br />
newly emerging Douglas-fi r needles, where<br />
larvae feeding will damage the needles and<br />
cause them to break. The trap uses sunlight<br />
to attract the midge emerging from their<br />
overwintering spots in the soil at the base of<br />
Douglas-fi r trees.<br />
Trap Materials (Fig. 12)<br />
• Waxy cardboard box (approximately<br />
12–15 inches high and wide)<br />
• Clear plastic or glass jar (peanut butter jar<br />
works well)<br />
• Duct tape<br />
• Paper towel<br />
• Rocks or other weights<br />
Trap Assembly (Fig. 13)<br />
1. On one side of the box, near the bottom<br />
of the box (away from the opening),<br />
cut a hole slightly smaller than the<br />
mouth of the jar.<br />
2. Crumple the paper towel and put in<br />
the jar to absorb moisture and make<br />
detection of midges easier.<br />
3. Screw the jar into hole in the box and<br />
secure with duct tape.<br />
Using the Traps<br />
• Well be<strong>for</strong>e bud break, select a tree that<br />
had damage in previous year. If necessary,<br />
butt-prune the tree to allow <strong>for</strong> placement<br />
of the trap.<br />
• Place at least three traps per fi eld on the<br />
south side of trees.<br />
• Turn the box upside down so the open<br />
side is on the ground. Place it against the<br />
trunk on the southern exposure of the<br />
tree.<br />
• Secure the box in place with the rocks or<br />
other weights on the fl aps of box.<br />
• Midges that emerge from the soil under<br />
the box will fl y to the light/jar and collect<br />
there (Fig. 14).<br />
• As bud break approaches, monitor<br />
traps daily. Egg laying occurs very soon<br />
after emergence, so it is important that<br />
very little time elapses between the fi rst<br />
sign of midges in traps and a pesticide<br />
application.<br />
• If midges are found in the traps, empty<br />
the jar and destroy the midges be<strong>for</strong>e<br />
replacing the jar.<br />
Figure 14. Midges collected in the jar; a paper<br />
towel in the jar would help prevent condensation.<br />
Courtesy of Sandy Gardosik, PDA<br />
APPENDIX E .......................................................................................................................................................................................................................................................................................... 158
Bagworm Traps<br />
Bagworm larvae feed on the foliage of many<br />
conifer and hardwood tree species. These<br />
lure-baited sticky traps (Figs. 15 and 16)<br />
monitor the late summer emergence of adult<br />
male bagworm moths. Trapping helps reduce<br />
the population of breeding males and,<br />
consequently, the number of overwintering<br />
eggs.<br />
Figure 15.<br />
Bagworm sticky<br />
trap and lure.<br />
Courtesy of<br />
Sarah Pickel,<br />
PDA<br />
Figure 16. Adult male bagworm moth (and<br />
other insects) caught in sticky trap. Courtesy of<br />
Tracey Olson, PDA<br />
Ordering<br />
Bagworm traps can be ordered from Great<br />
Lakes IPM. The kit includes one trap top,<br />
one wire hanger with plastic separators, two<br />
sticky trap bottoms, and one lure (will last<br />
entire season), is listed as catalog # IPM-10,<br />
and sells <strong>for</strong> $3.70 (2010 catalog).<br />
The bagworm lure, listed as catalog<br />
# IPM-BGW, may be purchased separately<br />
<strong>for</strong> $1.55 each (2010 catalog).<br />
Using the Trap<br />
• Assemble the traps following the<br />
instructions provided with the trap.<br />
• Use two to four traps per acre in areas<br />
where bagworm infestations have been<br />
found.<br />
• Traps should be placed 10–15 feet away<br />
from the tree block being monitored.<br />
Hang traps 4–5 feet off the ground.<br />
• Begin monitoring with traps at the<br />
beginning of August.<br />
• <strong>Pest</strong>icide or Bt applications should not<br />
be made until the following summer and<br />
then only when young bagworm larvae<br />
are found exiting the bags.<br />
INSECT TRAP USE AND CONSTRUCTION ............................................................................................................................................................................................................................................. 159
APPENDIX F<br />
SCOUTING RECORD TEMPLATE<br />
FOR COMMON PINE PESTS<br />
Regularly scouting a tree farm <strong>for</strong> pest activity is a<br />
major part of an integrated pest management program.<br />
Along with scouting, keeping accurate records<br />
of scouting observations is essential. When scouting<br />
observations are recorded each year, they can serve<br />
as an important reference <strong>for</strong> future growing seasons.<br />
The charts on the next two pages are data-recording<br />
tools to be used by growers when scouting in fi elds<br />
of various pines. These charts can be copied back to<br />
back and kept in a fi eld notebook, where observations<br />
from each scouting trip can be recorded. The<br />
farm/fi eld names and descriptions can be listed on<br />
the second chart and will serve as a reference <strong>for</strong> the<br />
fi rst chart. <strong>Pest</strong>s common to pine species are listed<br />
across the chart, and below each pest several life<br />
stages and events are described. Growers may fi ll in<br />
the chart using the abbreviations given in the key<br />
below the chart.<br />
APPENDIX F ........................................................................................................................................................................................................................................................................................... 160
Scouting Record Template <strong>for</strong> Common Pine <strong>Pest</strong>s<br />
White Pine<br />
Weevil<br />
Striped<br />
Pine<br />
Scale<br />
Pine Needle<br />
Scale<br />
Pine Bark<br />
Adelgid<br />
Needle Casts<br />
of Pines*<br />
Eriophyid<br />
Sheath Mites<br />
Bud<br />
Break<br />
Bagworm<br />
Emergence holes<br />
Leader wilting<br />
Feeding punctures/adults on leader<br />
Adults in trap<br />
Crawlers emerged<br />
Eggs inside female covering<br />
Second-generation crawlers emerged<br />
First-generation crawlers emerged<br />
Overwintering eggs<br />
Nymphs with white waxy fringe<br />
Overwintering nymphs<br />
Fruiting bodies/needle surface ruptured<br />
Yellowing of current year’s growth<br />
Eriophyid activity<br />
Eggs hatched<br />
Overwintering eggs<br />
Buds broken or shoots elongating (%)<br />
Bags present<br />
Larvae emerging<br />
Eggs present<br />
Farm/Field Name<br />
(complete reverse<br />
<strong>for</strong> each block)<br />
Date<br />
3/14/09 Smith/Block 4 Y 0% Y N ? Y N ?<br />
*Send these disease samples to PDA or Penn State <strong>for</strong> diagnosis.<br />
Y = <strong>Pest</strong> stage/damage found during scouting<br />
N = <strong>Pest</strong> stage/damage not present<br />
? = Did not scout <strong>for</strong> pest<br />
Please complete in<strong>for</strong>mation on reverse.
Scouting Record Template <strong>for</strong> Common Pine <strong>Pest</strong>s (continued)<br />
Please provide in<strong>for</strong>mation <strong>for</strong> all farms and fi elds that have any species of pine planted.<br />
This in<strong>for</strong>mation will be used to reference the fi eld during scouting activities listed on the front of this record.<br />
Farm/Field Pine Species Description (age, condition, etc.) Notes<br />
Smith/Field 4 Eastern white pine Planted 2004 as 2:0 from The Best Seedling Nursery History of bagworm, white pine sheath mite, and white pine weevil
APPENDIX G<br />
SCOUTING RECORD TEMPLATE<br />
FOR COMMON SPRUCE PESTS<br />
Regularly scouting a tree farm <strong>for</strong> pest activity is<br />
a major part of an integrated pest management<br />
program. Along with scouting, accurate record<br />
keeping of scouting observations is essential.<br />
When scouting observations are recorded each<br />
year, they can serve as an important reference <strong>for</strong><br />
future growing seasons. The charts on the next<br />
two pages are data-recording tools to be used by<br />
growers when scouting in fi elds of various spruces.<br />
These charts can be copied back to back and<br />
kept in a fi eld notebook, where observations from<br />
each scouting trip can be recorded. The farm/fi eld<br />
names and descriptions can be listed on the second<br />
chart and will serve as a reference <strong>for</strong> the fi rst<br />
chart. <strong>Pest</strong>s common to spruce species are listed<br />
across the chart, and below each pest several life<br />
stages and events are described. Growers may fi ll in<br />
the chart using the abbreviations given in the key<br />
below the chart.<br />
APPENDIX G .......................................................................................................................................................................................................................................................................................... 164
Scouting Record Template <strong>for</strong> Common Spruce <strong>Pest</strong>s<br />
White Pine<br />
Weevil<br />
Spruce<br />
Spider Mite<br />
Spruce<br />
Needle Rust<br />
Eriophyid<br />
Rust Mites<br />
Eastern Spruce<br />
Gall Adelgid<br />
Cooley Spruce<br />
Gall Adelgid<br />
Bud<br />
Break<br />
Bagworm<br />
Emergence holes<br />
Leader wilting<br />
Feeding punctures/ adults on leader<br />
Adults in trap<br />
>10 mites/sample unit<br />
Egg hatch<br />
Overwintering eggs<br />
Sporulation<br />
Symptoms<br />
Eriophyid activity<br />
Egg hatch<br />
Overwintering eggs<br />
Galls <strong>for</strong>ming<br />
Nymphs with white waxy fringe<br />
Overwintering nymphs<br />
Galls <strong>for</strong>ming<br />
Nymphs with white waxy fringe<br />
Overwintering nymphs<br />
Buds broken or shoots elongating (%)<br />
Bags present<br />
Larvae emerging<br />
Eggs present<br />
Farm/Field Name<br />
(complete reverse<br />
<strong>for</strong> each block)<br />
Date<br />
Y = <strong>Pest</strong> stage/damage found during scouting<br />
N = <strong>Pest</strong> stage/damage not present<br />
? = Did not scout <strong>for</strong> pest<br />
Please complete in<strong>for</strong>mation on reverse.
Scouting Record Template <strong>for</strong> Common Spruce <strong>Pest</strong>s (continued)<br />
Please provide in<strong>for</strong>mation <strong>for</strong> all farms and fi elds that have any species of spruce planted.<br />
This in<strong>for</strong>mation will be used to reference the fi eld during scouting activities listed on the front of this record.<br />
Farm/Field Spruce Species Description (age, condition, etc.) Notes
APPENDIX H<br />
SCOUTING RECORD TEMPLATE<br />
FOR COMMON TRUE FIR<br />
AND DOUGLAS-FIR PESTS<br />
Regularly scouting a tree farm <strong>for</strong> pest activity is<br />
a major part of an integrated pest management<br />
program. Along with scouting, accurate record<br />
keeping of scouting observations is essential. When<br />
scouting observations are recorded each year, they<br />
can serve as an important reference <strong>for</strong> future growing<br />
seasons. The charts on the next two pages are<br />
data-recording tools to be used by growers when<br />
scouting in fi elds of various true fi rs and Douglas-fi r.<br />
These charts can be copied back to back and kept<br />
in a fi eld notebook, where observations from each<br />
scouting trip can be recorded. The farm/fi eld names<br />
and descriptions can be listed on the second chart<br />
and will serve as a reference <strong>for</strong> the fi rst chart. <strong>Pest</strong>s<br />
common to true fi rs and Douglas-fi r species are<br />
listed across the chart, and below each pest several<br />
life stages and events are described. Growers may<br />
fi ll in the chart using the abbreviations given in the<br />
key below the chart.<br />
APPENDIX H .......................................................................................................................................................................................................................................................................................... 168
Scouting Record Template <strong>for</strong> Common True Fir and Douglas-Fir <strong>Pest</strong>s<br />
White Pine<br />
Weevil<br />
Spruce<br />
Spider Mite<br />
Eriophyid<br />
Rust Mites<br />
Elongate<br />
Hemlock<br />
Scale<br />
Cryptomeria<br />
Scale<br />
Cooley<br />
Spruce<br />
Gall<br />
Adelgid<br />
Bud<br />
Break<br />
Balsam<br />
Wooly<br />
Adelgid<br />
Balsam<br />
Twig<br />
Aphid<br />
Bagworm<br />
Emergence holes<br />
Leader wilting<br />
Feeding punctures/adults on leader<br />
Adults in trap<br />
>10 mites/sample unit<br />
Egg hatch<br />
Overwintering eggs<br />
Eriophyid activity<br />
Egg hatch<br />
Overwintering eggs<br />
Crawlers emerged<br />
Eggs inside female scales<br />
Second-generation crawlers emerged<br />
First-generation crawlers emerged<br />
Eggs observed under adult<br />
Nymphs with white waxy fringe<br />
Overwintering nymphs<br />
Buds broken or shoots elongating (%)<br />
Nymphs with white waxy fringe<br />
Overwintering nymphs<br />
Stem mothers hatched<br />
Overwintering eggs<br />
Bags present<br />
Larvae emerging<br />
Eggs present<br />
Farm/Field<br />
Name (complete<br />
reverse <strong>for</strong> each<br />
block)<br />
Date<br />
Y = <strong>Pest</strong> stage/damage found during scouting<br />
N = <strong>Pest</strong> stage/damage not present<br />
? = Did not scout <strong>for</strong> pest<br />
Please complete in<strong>for</strong>mation on reverse.
Scouting Record Template <strong>for</strong> Common True Fir and Douglas-Fir <strong>Pest</strong>s (continued)<br />
Please provide in<strong>for</strong>mation <strong>for</strong> all farms and fi elds that have any species of true fi r and Douglas-fi r planted.<br />
This in<strong>for</strong>mation will be used to reference the fi eld during scouting activities listed on the front of this record.<br />
Farm/Field Fir Species Description (age, condition, etc.) Notes
APPENDIX I<br />
GROWING DEGREE DAY AND<br />
TEMPERATURE RECORD TEMPLATE<br />
Tracking growing degree days, or units of daily heat<br />
accumulation, is a way to pinpoint the timing of<br />
certain insect life cycle events—specifi cally, life stages<br />
when insects are vulnerable to chemical controls.<br />
This process involves recording daily temperatures<br />
and using a simple equation to convert these temperatures<br />
into growing degree days. The following<br />
chart explains the simple equation and can be copied<br />
and used as a record <strong>for</strong> these daily temperatures and<br />
growing degree day fi gures. A space <strong>for</strong> daily ground<br />
temperatures is also provided. This data is useful when<br />
monitoring <strong>for</strong> white pine weevil, a common pest<br />
throughout the country. Other daily weather in<strong>for</strong>mation<br />
may also be useful to growers, so space is provided<br />
<strong>for</strong> that data. For more in<strong>for</strong>mation on growing degree<br />
day and temperature monitoring, refer to page 18 of<br />
the IPM Basics section.<br />
APPENDIX I ........................................................................................................................................................................................................................................................................................... 172
Growing Degree Day and Temperature Record Template<br />
Remember to only record positive numbers.<br />
Ground temperature is helpful when monitoring <strong>for</strong> white pine weevil.<br />
Insert probe thermometer 2 inches into soil and look <strong>for</strong> 50°F to signal<br />
adult weevil emergence.<br />
GDD <strong>for</strong>mula:<br />
Low Temperature + High Temperature – 50°F = GDD<br />
2<br />
Example 1:<br />
46°F + 60°F = 106 = 53 – 50 = 3 Add to GDD running total.<br />
2 2<br />
Example 2:<br />
35°F + 55°F = 90 = 45 – 50 = -5 Don’t add to GDD running total.<br />
2 2<br />
Farm: ______________________________________________________________________ Field: _____________________________________________________________________<br />
Air Temperature<br />
Low High Avg. Daily GDD GDD Running Total Ground Temperature Other Observations (rainy, sunny, snow cover, etc.)<br />
Collector’s<br />
Initials<br />
Date Time
APPENDIX J<br />
PESTICIDE APPLICATION<br />
RECORD TEMPLATE<br />
The chart that follows is a template <strong>for</strong> growers<br />
to copy and record pesticide application in<strong>for</strong>mation<br />
<strong>for</strong> their farms. Under the law, private<br />
applicators must keep records on applications<br />
of restricted-use pesticides. On farms where<br />
workers are employed, records must be kept<br />
on all pesticide applications, according to the<br />
federal Worker Protection Standard. Additionally,<br />
commercial/public applicators must keep<br />
records <strong>for</strong> every application they make.<br />
APPENDIX J ........................................................................................................................................................................................................................................................................................... 174
<strong>Pest</strong>icide Application Record Template<br />
Establishment Name: ___________________________________________________ Address: ___________________________________________________________________<br />
Restricted-Use <strong>Pest</strong>icide and Worker Protection Application Records<br />
Application<br />
Rate<br />
EPA Reg.<br />
Number<br />
Start Time<br />
Applicator’s Name<br />
and Certifi cation<br />
Number<br />
Completion<br />
Time<br />
Quantity of<br />
Undiluted<br />
<strong>Pest</strong>icide Used<br />
Size of<br />
Treated Area<br />
Site, Crop, or<br />
Commodity<br />
Treated<br />
Active<br />
Ingredient<br />
<strong>Pest</strong>icide Brand<br />
Name and<br />
Formulation<br />
Application Site Location/<br />
Description<br />
REI (hrs)<br />
Date<br />
AM<br />
PM AM<br />
PM<br />
AM<br />
PM AM<br />
PM<br />
AM<br />
PM AM<br />
PM<br />
AM<br />
PM AM<br />
PM<br />
AM<br />
PM AM<br />
PM<br />
AM<br />
PM AM<br />
PM
APPENDIX K<br />
CONTACT INFORMATION<br />
This appendix contains in<strong>for</strong>mation<br />
pertaining to biological controls, IPM<br />
supplies, pest management supplies,<br />
and other equipment. This is an<br />
incomplete list, so no discrimination<br />
is intended and no endorsement<br />
by the Pennsylvania Department of<br />
Agriculture, Penn State Cooperative<br />
Extension, or Penn State’s College<br />
of Agricultural Sciences is implied.<br />
Every ef<strong>for</strong>t has been made to provide<br />
correct and up-to-date in<strong>for</strong>mation.<br />
APPENDIX K .......................................................................................................................................................................................................................................................................................... 176
Association of Natural Biocontrol<br />
Producers (ANBP)<br />
PO Box 1609<br />
Clovis, CA 93613-1609<br />
Phone: 559-360-7111<br />
Fax: 800-553-4817<br />
www.anbp.org/index.htm<br />
The National <strong>Christmas</strong> <strong>Tree</strong> Association<br />
16020 Swingley Ridge Road, Suite 300<br />
Chesterfi eld, MO 63017<br />
Members/<strong>Christmas</strong> <strong>Tree</strong> Industry:<br />
636-449-5070<br />
News Media: 636-449-5071<br />
Fax: 636-449-5051<br />
E-mail: info@realchristmastrees.org<br />
www.christmastree.org/home.cfm<br />
Pennsylvania <strong>Christmas</strong> <strong>Tree</strong> Growers<br />
Association<br />
4305 North Sixth Street, Suite A<br />
Harrisburg, PA 17110<br />
Toll-free: 800-547-2842<br />
Phone: 717-238-9765<br />
Fax: 717-238-9985<br />
E-mail: info@christmastrees.org<br />
www.christmastrees.org<br />
Pennsylvania Landscape and Nursery<br />
Association (PLNA)<br />
1707 South Cameron Street<br />
Harrisburg, PA 17104-3148<br />
Toll-free: 800-898-3411<br />
Phone: 717-238-1673<br />
Fax: 717-238-1678<br />
E-mail: plna@plna.com<br />
www.plna.com<br />
Pennsylvania Department<br />
of Agriculture<br />
Sandy Gardosik<br />
Entomologist<br />
2301 North Cameron Street<br />
Harrisburg, PA 17110<br />
Phone: 717-772-0521<br />
E-mail: sgardosik@state.pa.us<br />
Tracey Olson<br />
Plant Pathologist<br />
2301 North Cameron Street<br />
Harrisburg, PA 17110<br />
Phone: 717-783-9636<br />
E-mail: tolson@state.pa.us<br />
Sarah Pickel<br />
Education Specialist<br />
2301 North Cameron Street<br />
Harrisburg, PA 17110<br />
717-772-5227 Phone:<br />
E-mail: c-sapickel@state.pa.us<br />
Region I Offi ce<br />
13410 Dunham Road<br />
Meadville, PA 16335-8346<br />
Phone: 814-332-6890<br />
Region II Offi ce<br />
542 County Farm Road, Suite 102<br />
Montoursville, PA 17754-9685<br />
Phone: 570-433-2640<br />
Region III Offi ce<br />
Route 92 South, PO Box C<br />
Tunkhannock, PA 18657-0318<br />
Phone: 717-570-2181<br />
Region IV Offi ce<br />
6 McIntyre Road<br />
Gibsonia, PA 15044-9644<br />
Phone: 724-443-1585<br />
Region V Offi ce<br />
1307 7th Street, Cricketfi eld Plaza<br />
Altoona, PA 16601-4701<br />
Phone: 814-946-7315<br />
Region VI Offi ce<br />
PO Box 5184<br />
Harrisburg, PA 17110-0184<br />
Phone: 717-346-3223<br />
Region VII Offi ce<br />
Route 113, PO Box 300<br />
Creamery, PA 19430-0300<br />
Phone: 610-489-1003<br />
Brian Schildt<br />
IPM Scouting Consultant<br />
2301 North Cameron Street<br />
Harrisburg, PA 17110<br />
Phone: 717-772-5202<br />
E-mail: c-brschild@state.pa.us<br />
Cathy Thomas<br />
IPM Coordinator<br />
2301 North Cameron Street<br />
Harrisburg, PA 17110<br />
Phone: 717-772-5204<br />
E-mail: caththomas@state.pa.us<br />
CONTACT INFORMATION ..................................................................................................................................................................................................................................................................... 177
The Pennsylvania State University<br />
Ricky Bates<br />
Assistant Professor of Ornamental<br />
Horticulture<br />
303 Tyson Building<br />
University Park, PA 16802<br />
Phone: 814-863-2198<br />
E-mail: rmb30@psu.edu<br />
Andrew Beck<br />
Extension Educator, Schuylkill and Berks<br />
Counties<br />
1202 Ag Center Drive<br />
Pottsville, PA 17901<br />
Phone: 570-622-4225<br />
E-mail: awb123@psu.edu<br />
Tom Butzler<br />
Extension Educator, Clinton County<br />
47 Cooperation Lane<br />
Mill Hall, PA 17751-8978<br />
Phone: 570-726-0022<br />
E-mail: tmb124@psu.edu<br />
Kerry Hoffman-Richards<br />
Acting Director<br />
Penn State <strong>Pest</strong>icide Education Program<br />
114 Buckhout Lab<br />
University Park, PA 16802<br />
Phone: 814-865-2134<br />
E-mail: kmh14@psu.edu<br />
Greg Hoover<br />
Senior Extension Associate, Entomology<br />
543 ASI Building<br />
University Park, PA 16802<br />
Phone: 814-865-3256<br />
E-mail: gah10@psu.edu<br />
Larry Kuhns<br />
Professor Emeritus of Horticulture<br />
304 Tyson Building<br />
University Park, PA 16802<br />
Phone: 814-863-2197<br />
E-mail: ljk@psu.edu<br />
Robert Pollock<br />
Extension Educator, Indiana County<br />
827 Water Street<br />
Indiana, PA 15701-1765<br />
Phone: 724-465-3880<br />
E-mail: rcp3@psu.edu<br />
Suppliers<br />
Abraczinskas Nurseries, Inc.<br />
346 Numidia Drive<br />
Catawissa, PA 17820<br />
Phone: 570-356-2323<br />
Fax: 570-356-2366<br />
E-mail: info@abraczinskas.com<br />
www.abraczinskas.com<br />
B & G Equipment Company<br />
135 Region South Drive<br />
Jackson, GA 30233<br />
Phone: 678-688-5601<br />
Fax: 678-688-5633<br />
www.bgequip.com/index.html<br />
Ben Meadows Company<br />
PO Box 5277<br />
Janesville, WI 53547-5277<br />
Toll-free: 800-241-6401<br />
Fax: 800-628-2068<br />
www.benmeadows.com/home.htm<br />
Berkey’s Nursery<br />
138 Jefferson Street<br />
PO Box 215<br />
Spartansburg, PA 16434<br />
Toll-free: 800-203-9210<br />
Fax: 814-654-7503<br />
E-mail: info@berkeysnursery.com<br />
www.berkeysnursery.com<br />
BioQuip Products<br />
2321 Gladwick Street<br />
Rancho Dominguez, CA 90220<br />
Phone: 310-667-8800<br />
Fax: 310-667-8808<br />
www.bioquip.com<br />
Carl Neutzel Services, Inc.<br />
2648 Openshaw Road<br />
White Hall, MD 21161<br />
Toll-free: 888-580-5325<br />
Phone: 410-329-6791<br />
Fax: 410-357-4175<br />
E-mail: cwneutzel@verizon.net<br />
carlneutzel.com<br />
Earl F. Kegerise Inc<br />
3454 Pricetown Road<br />
Fleetwood, PA 19522<br />
Phone: 610-944-8532<br />
E-mail: larrykeg@aol.com<br />
Forestry Suppliers Inc.<br />
205 West Rankin Street<br />
PO Box 8397<br />
Jackson, MS 39284-8397<br />
Toll-free: 800-647-5368<br />
www.<strong>for</strong>estry-suppliers.com/index1.asp<br />
APPENDIX K .......................................................................................................................................................................................................................................................................................... 178
Gardens Alive!, Inc.<br />
5100 Schenley Place<br />
Lawrenceburg, IN 47025<br />
Phone: 513-354-1482<br />
www.gardensalive.com/default.asp<br />
Gempler’s<br />
PO Box 44993<br />
Madison, WI 53744-4993<br />
Toll-free: 800-382-8473<br />
E-mail: customerservice@gemplers.com<br />
www.gemplers.com<br />
Great Lakes IPM, Inc.<br />
10220 Church Rd NE<br />
Vestaburg, MI 48891<br />
Toll-free: 800-235-0285<br />
Phone: 989-268-5693<br />
Phone: 989-268-5911<br />
www.greatlakesipm.com<br />
The Green Spot, Ltd.<br />
93 Priest Road<br />
Nottingham, NH 03290-6204<br />
Phone: 603-942-8925<br />
Fax: 603-942-8932<br />
greenmethods.com/site<br />
Growquest Growers<br />
134 Davis Street<br />
Santa Paula, CA 93060<br />
Phone: 805-921-3900<br />
E-mail: sales@growquest.com<br />
www.growquest.com<br />
H. D. Hudson Manufacturing Company<br />
(Hudson Sprayers and Dusters)<br />
500 N. Michigan Avenue<br />
Chicago, IL 60611-3669<br />
Toll-free: 800-977-7293<br />
E-mail: ladybug@hdhudson.com<br />
www.hdhudson.com<br />
Hummert International<br />
4500 Earth City Expressway<br />
Earth City, MO 63045<br />
Toll-free: 800-325-3055<br />
E-mail: sales@hummert.com<br />
www.hummert.com<br />
Insects Limited, Inc.<br />
16950 Westfi eld Park Road<br />
Westfi eld, IN 46074<br />
Phone: 317-896-9300<br />
Fax: 317-867-5757<br />
www.insectslimited.com<br />
International Technology Services, Inc.<br />
Toll-free: 877-505-9703<br />
Phone: 303-661-9546<br />
Fax: 303-552-5747<br />
www.intertechserv.com<br />
IPM Laboratories, Inc.<br />
980 Main Street<br />
Locke, NY 13092<br />
Phone: 315-497-2063<br />
Fax: 315-497-3129<br />
E-mail: ipminfo@ipmlabs.com<br />
ipmlabs.com/home.php<br />
Koch’s Lawn and Garden, Inc.<br />
1044 Summer Valley Road<br />
PO Box 232<br />
New Ringgold, PA 17960<br />
Phone: 570-943-2367<br />
Fax: 570-943-7767<br />
www.kochslg.com<br />
Koppert Biological Systems, Inc.<br />
28465 Beverly Road<br />
Romulus, MI 48174<br />
Toll-free: 800-928-8827<br />
Fax: 734-641-3799<br />
E-mail: info@koppertonline.com<br />
www.koppertonline.com/home.asp<br />
Martin’s Repair Shop, LLC<br />
28 East Trout Run Road<br />
Ephrata, PA 17522<br />
Phone: 717-733-3015<br />
Fax: 717-733-9619<br />
E-mail: martinsreshllc@characterlink.net<br />
Peaceful Valley Farm Supply, Inc.<br />
125 Clydesdale Court<br />
PO Box 2209<br />
Grass Valley, CA 95945<br />
Phone: 888-784-1722<br />
Phone: 530-272-4769<br />
E-mail: helpdesk@groworganic.com<br />
www.groworganic.com/default.html<br />
Rincon-Vitova Insectaries<br />
PO Box 1555<br />
Ventura, CA 93002-1555<br />
Toll-free: 800-248-2847<br />
www.rinconvitova.com/index.htm<br />
Scentry Biologicals, Inc.<br />
610 Central Avenue<br />
Billings, MT 59102<br />
Toll-free: 800-735-5323<br />
Phone: 406-245-3016<br />
Fax: 406-245-2790<br />
E-mail: customerservice@scentry.com<br />
www.scentry.com<br />
CONTACT INFORMATION ..................................................................................................................................................................................................................................................................... 179
Suppliers (continued)<br />
Sheerlund Products<br />
740 Corporate Drive<br />
Reading, PA 19605<br />
Toll-free: 800-233-2958<br />
www.sheerlundproducts.com/store/home<br />
.php<br />
Solo<br />
5100 Chestnut Avenue<br />
Newport News, VA 23605<br />
Phone: 757-245-4228<br />
Fax: 757-245-0800<br />
www.solousa.com/store<br />
Syngenta Bioline, Inc.<br />
PO Box 2430<br />
Oxnard, CA 93034-2430<br />
Phone: 805-986-8265<br />
Fax: 805-986-8267<br />
E-mail: Dcahn@syngentabioline.com<br />
www.syngenta-bioline.co.uk/Default.html<br />
Trécé, Inc.<br />
7569 Highway 28 West<br />
PO Box 129<br />
Adair, OK 74330<br />
Phone: 918-785-3061<br />
Fax: 918-785-3063<br />
E-mail: custserv@trece.com<br />
www.trece.com<br />
<strong>Tree</strong>-Teck, Inc.<br />
1773 Long Run Road<br />
Schuylkill Haven, PA 17972<br />
Toll-free: 800-477-8637<br />
Phone: 570-366-4997<br />
E-mail: info@treeteck.com<br />
www.treeteck.com/about.html<br />
<strong>Tree</strong>wheeler (Vandervalk <strong>Tree</strong> Farm)<br />
25 Lovell Street<br />
Mendon, MA 01756<br />
Phone: 508-478-8733<br />
www.treewheeler.com/treewheeler.html<br />
APPENDIX K .......................................................................................................................................................................................................................................................................................... 180
CONTACT INFORMATION ..................................................................................................................................................................................................................................................................... 181
APPENDIX L<br />
LISTS OF PESTS<br />
All of the pests that are detailed in this<br />
manual are listed on the following two<br />
pages. The fi rst list organizes the pests<br />
alphabetically by common name and<br />
includes scientifi c names and the pages of<br />
the corresponding fact sheets. The second<br />
list groups the pests by their conifer<br />
hosts. Several of these pests have multiple<br />
conifer hosts, so they may be repeated in<br />
the list.<br />
APPENDIX L ........................................................................................................................................................................................................................................................................................... 182
PESTS IN ALPHABETICAL ORDER<br />
Common Name Scientifi c Name Page<br />
Armillaria root rot Armillaria spp. 107<br />
Atropellis canker Atropellis tingens Lohman and Cash (also A. apiculata Lohman, Cash, and R. W.<br />
Davidson, A. pinicola Zeller and Goodd, and A. piniphila [Weir] Lohman and Cash) 73<br />
Bagworm Thyridopteryx ephemerae<strong>for</strong>mis (Haworth) 23<br />
Balsam twig aphid Mindarus abietinus Koch 26<br />
Balsam woolly adelgid Adelges piceae (Ratzeburg) 75<br />
Bark and engraver beetles Ips spp. and Pityogenes hopkinsi Swaine 109<br />
Botrytis blight Botrytis cinerea Pers.: Fr. 78<br />
Cooley spruce gall adelgid Adelges cooleyi (Gillette) 29, 80<br />
Cryptomeria scale Aspidiotus cryptomeriae Kuwana 31<br />
Cyclaneusma needle cast Cyclaneusma minus (Butin) DiCosmo, Peredo, and Minter<br />
(syn. Naemacyclus minor Butin) 34<br />
Diplodia (Sphaeropsis) tip blight Diplodia pinea (Desm.) J. Kickx f. 82<br />
Douglas-fi r needle midge Contarinia pseudotsugae Condrashoff 37<br />
Eastern (pine-oak) gall rust Cronartium quercuum (Berk.) Miyabe ex Shirai 88<br />
Eastern pine weevil Pissodes nemorensis Germar 84<br />
Eastern spruce gall adelgid Adelges abietis (Linnaeus) 86<br />
Elongate hemlock scale Fiorinia externa Ferris 39<br />
Eriophyid rust and sheath mites Nalepella and Setoptus spp. 42<br />
European pine sawfl y Neodiprion sertifer (Geoffroy) 53<br />
Gypsy moth Lymantria dispar (Linnaeus) 45<br />
Introduced pine sawfl y Diprion similis (Hartig) 53<br />
Japanese beetle Popillia japonica Newman 118<br />
Lophodermium needle cast Lophodermium seditiosum Minter, Staley, and Millar 48<br />
Pales weevil Hylobius pales (Herbst) 90<br />
Phytophthora root rot Phytophthora spp. 111<br />
Pine bark adelgid Pineus strobi (Hartig) 92<br />
Pine needle scale Chionaspis pinifoliae (Fitch) 51<br />
Pine root collar weevil Hylobius radicis Buchanan 113<br />
Pine shoot beetle Tomicus piniperda (Linnaeus) 94<br />
Pine wilt disease Caused by pinewood nematode,<br />
Bursaphelenchus xylophilus (Steiner and Buhrer) (Nickle) 116<br />
Ploioderma needle cast Ploioderma lethale (Dearn.) Darker 56<br />
Red-band (Dothistroma) needle blight Mycosphaerella pini Rostr.<br />
Anamorph: Dothistroma septospora (Doroguine) Morelet 58<br />
Redheaded pine sawfl y Neodiprion lecontei (Fitch) 53<br />
Rhabdocline needle cast Rhabdocline weirii A. K. Parker and J. Reid,<br />
Rhabdocline pseudotsugae Syd. 60<br />
Rhizosphaera needle cast Rhizosphaera kalkhoffi i Bubak 63<br />
Spruce needle rust Chrysomyxa weirii (H. S. Jacks) 65<br />
Spruce spider mite Oligonychus ununguis (Jacobi) 67<br />
Striped pine scale Toumeyella pini (King) 97<br />
Swiss needle cast Phaeocryptopus gäumannii (T. Rohde) Petr. 70<br />
Western (pine-pine) gall rust Endocronartium harknessii (J. P. Moore) Y. Hiratsuka 88<br />
White grubs (May, June, and Japanese beetles) Phyllophaga sp., Polyphylla sp., Popillia japonica Newman 118<br />
White pine blister rust Cronartium ribicola J. C. Fisch 99<br />
White pine weevil Pissodes strobi (Peck) 101<br />
Zimmerman pine moth Dioryctria zimmermani (Grote) 120<br />
LISTS OF PESTS ..................................................................................................................................................................................................................................................................................... 183
PESTS BY HOST GENUS<br />
<strong>Tree</strong> Genus <strong>Pest</strong>s<br />
Douglas-fi r (Pseudotsuga) Armillaria root rot, bagworm, Botrytis blight, Cooley spruce gall<br />
adelgid, Cryptomeria scale, Diplodia (Sphaeropsis) tip blight,<br />
Douglas-fi r needle midge, eastern pine weevil, elongate hemlock<br />
scale, gyps moth, Japanese beetle, pales weevil, Phytophthora root<br />
rot, pine needle scale, Rhabdocline needle cast, spruce spider mite,<br />
Swiss needle cast, white grubs, white pine weevil, Zimmerman pine<br />
moth<br />
Fir (Abies) Armillaria root rot, bagworm, balsam twig aphid, balsam woolly<br />
adelgid, Botrytis blight, Cryptomeria scale, elongate hemlock scale,<br />
eriophyid mites, gypsy moth, Japanese beetle, pales weevil, Phytoph-<br />
thora root rot, pine needle scale, spruce spider mite, white grubs,<br />
white pine weevil<br />
Pines (Pinus) Armillaria root rot, Atropellis canker, bagworm, bark and engraver<br />
beetles, Botrytis blight, Cryptomeria scale, Cyclaneusma needle cast,<br />
Diplodia (Sphaeropsis) tip blight, eastern pine weevil, eriophyid mites,<br />
gall rusts, gypsy moth, Japanese beetle, Lophodermium needle cast,<br />
pales weevil, Phytophthora root rot, pine bark adelgid, pine needle<br />
scale, pine root collar weevil, pine sawfl ies, pine shoot beetle, Ploio-<br />
derma needle cast, red-band needle blight, spruce spider mite, striped<br />
pine scale, white grubs, white pine blister rust, white pine weevil,<br />
Zimmerman pine moth<br />
Spruce (Picea) Armillaria root rot, bagworm, Botrytis blight, Cooley spruce gall<br />
adelgid, Cryptomeria scale, Diplodia (Sphaeropsis) tip blight, eastern<br />
pine weevil, eastern spruce gall adelgid, elongate hemlock scale,<br />
eriophyid mites, gypsy moth, Japanese beetle, pales weevil, Phytoph-<br />
thora root rot, pine needle scale, Rhizosphaera needle cast, spruce<br />
needle rust, spruce spider mite, white grubs, white pine weevil,<br />
Zimmerman pine moth<br />
APPENDIX L ........................................................................................................................................................................................................................................................................................... 184
BIBLIOGRAPHY
Bibliography<br />
General Sources<br />
Borror, D. J., D. M. DeLong, and C. A. Triplehorn. An Introduction to the Study of Insects.<br />
4th ed. New York: Holt, Rinehart, and Winston, 1976.<br />
Chastagner, G. A, ed. <strong>Christmas</strong> <strong>Tree</strong> Diseases, Insects, and Disorders in the Pacifi c Northwest:<br />
Identifi cation and <strong>Management</strong>. Puyallup: Washington State University Cooperative<br />
Extension, 1997.<br />
Hansen, E. M., and K. J. Lewis. Compendium of Conifer Diseases. St. Paul: APS Press, 1997.<br />
Hock, W. K., and C. L. Brown, eds. <strong>Pest</strong>icide Education Manual. 2nd ed. University Park:<br />
The Pennsylvania State University, 1992.<br />
Johnson, W. T., and H. H. Lyon. Insects That Feed on <strong>Tree</strong>s and Shrubs. 2nd ed. Ithaca:<br />
Cornell University Press, 1991.<br />
Kowalsick, T. “Using Growing Degree-Days <strong>for</strong> Insect <strong>Pest</strong> <strong>Management</strong>.” Ithaca: Cornell<br />
Cooperative Extension, 2008. ccesuffolk.org/assets/Horticulture-Leafl ets/Using-Growing-<br />
Degree-Days-<strong>for</strong>-Insect-<strong>Pest</strong>-<strong>Management</strong>.pdf.<br />
Krischik, V., and J. Davidson. “<strong>Pest</strong>s of <strong>Tree</strong>s and Shrubs.” In IPM of Midwest Landscapes,<br />
section IV. University of Minnesota, 2007. www.entomology.umn.edu/cues/IPM-trees/<br />
pests_in_trees.htm.<br />
• “Bagworm,” pp. 71–72. Accessed on December 18, 2008.<br />
• “Balsam Twig Aphid,” p. 73. Accessed on December 3, 2008.<br />
• “Cooley Spruce Gall Adelgid,” pp. 98–99. Accessed on December 9, 2009.<br />
• “Eastern Spruce Gall Adelgid,” p. 108. Accessed on January 9, 2009.<br />
• “Eriophyid Mites,” pp. 122–23. Accessed on January 13, 2009.<br />
• “European Pine Sawfl ies,” p. 128. Accessed on August 28, 2009.<br />
• “Introduced Pine Sawfl y,” p. 155. Accessed on August 28, 2009.<br />
• “Pales Weevil,” p. 175. Accessed on June 9, 2009.<br />
• “Pine Needle Scale,” pp. 181–82. Accessed on December 3, 2008.<br />
• “Redheaded Pine Sawfl y,” p. 192. Accessed on August 28, 2009.<br />
McCullough, D. G., S. A. Katovich, M. E. Ostry, and J. Cummings-Carlson, eds. <strong>Christmas</strong><br />
<strong>Tree</strong> <strong>Pest</strong> Manual. 2nd ed. East Lansing: Michigan State University Extension, 1998.<br />
Merrill, W., N. G. Wenner, and P. Heller. <strong>Christmas</strong> <strong>Tree</strong> <strong>Pest</strong> Notes. Department of Plant<br />
Pathology, College of Agricultural Sciences, The Pennsylvania State University, n.d.<br />
(out of print).<br />
Myers, T., ed. Mosby’s Medical Dictionary. 8th ed. St. Louis: Elsevier, 2009.<br />
Sinclair, W. A., and H. H. Lyon. Diseases of <strong>Tree</strong>s and Shrubs. 2nd ed. Ithaca: Cornell<br />
University Press, 2005.<br />
Wilson, L. F. A Guide to Insect Injury of Conifers in the Lake States. Agriculture Handbook<br />
No. 501. Washington, D.C.: USDA Forest Service, 1977.<br />
IPM FOR CHRISTMAS TREE PRODUCTION ......................................................................................................................................................................................................................................... 186
Technical Sources<br />
Armillaria Root Rot<br />
“Armillaria Root Rot.” Department of Plant Pathology, Cornell University. www.plantpath<br />
.cornell.edu/trees/Armillaria.html (accessed on March 10, 2009).<br />
“Armillaria Root Rot of <strong>Tree</strong>s and Shrubs.” University of Illinois Extension, Department of<br />
Crop Sciences. ipm.illinois.edu/diseases/rpds/602.pdf (accessed on March 10, 2009).<br />
Davidson, R. M., and R. S. Byther. “Armillaria (Shoestring) Root Rot (EB1776).”<br />
Washington State University Cooperative Extension. cru.cahe.wsu.edu/CEPublications/<br />
eb1776/eb1776.html (accessed on March 10, 2009).<br />
Atropellis Canker<br />
Blosser, W. E. “Atropellis Canker of Pine.” Plant Pathology Circular No. 81. Regulatory<br />
Horticulture 24, no. 1 (1998).<br />
Lightle, P. C., and J. H. Thompson. “Atropellis Canker of Pines.” Forest <strong>Pest</strong> Leafl et 138.<br />
Washington, D.C.: USDA Forest Service, 1973.<br />
Bagworm<br />
Barrett, B. A. “The Bagworm in Missouri.” University of Missouri Extension. extension<br />
.missouri.edu/explore/agguides/pests/g07250.htm (accessed on December, 18, 2008).<br />
Day, E. “Bagworm.” Virginia Polytechnic Institute and State University Cooperative<br />
Extension. www.ext.vt.edu/departments/entomology/factsheets/bagworm.html (accessed on<br />
December, 18, 2008).<br />
Hale, F. A., B. Klingeman, and K. M. Vail. “The Bagworm and Its Control.”<br />
University of Tennessee Institute of Agriculture. www.utextension.utk.edu/publications/<br />
spfi les/SP341-U.pdf (accessed on December 18, 2008).<br />
Mazzey, K., and M. Masiuk. “Woody Ornamental IPM: Bagworm.” Penn State Cooperative<br />
Extension. woodypests.cas.psu.edu/FactSheets/InsectFactSheets/pdf/Bagworm.pdf (accessed<br />
on December 18, 2008).<br />
Shetlar, D. J. “Bagworm and Its Control.” Ohio State University Extension Fact Sheet.<br />
ohioline.osu.edu/hyg-fact/2000/2149.html (accessed on December 18, 2008).<br />
Wheeler, A. G. Jr. “Bagworm, Thyridopteryx ephemerae<strong>for</strong>mis.” Entomology Circular No. 50.<br />
Regulatory Horticulture 6, no. 2 (1980): 9–12.<br />
Balsam Twig Aphid<br />
“Balsam Twig Aphid.” Center <strong>for</strong> Invasive Species and Ecosystem Health Bugwood<br />
Network, University of Georgia. www.<strong>for</strong>estpests.org/vermont/balsamtwig.html<br />
(accessed on November 20, 2008).<br />
Sidebottom, J. “Balsam Twig Aphid on Fraser Fir CTN-019.” North Carolina State University<br />
Cooperative Extension. www.ces.ncsu.edu/fl etcher/programs/xmas/ctnotes/ctn019.html<br />
(accessed on December 3, 2008).<br />
Stimmel, J. F. “Balsam Twig Aphid, Mindarus abietinus.” Entomology Circular No. 136.<br />
Regulatory Horticulture 16, no. 1 (1990): 25–26.<br />
BIBLIOGRAPHY .................................................................................................................................................................................................................................................................................... 187
Technical Sources (continued)<br />
Balsam Woolly Adelgid<br />
“Balsam Woolly Adelgid.” Center <strong>for</strong> Invasive Species and Ecosystem Health Bugwood<br />
Network, University of Georgia. www.<strong>for</strong>estpests.org/southern/balsamwoollyadelgid.html<br />
(accessed on April 13, 2009).<br />
Day, E. “Balsam Woolly Adelgid.” Virginia Polytechnic Institute and State University<br />
Cooperative Extension, 1996. www.ext.vt.edu/departments/entomology/factsheets/balwoade<br />
.html (accessed on April 13, 2009).<br />
Merrill W., and N. G. Wenner. “Balsam Woolly Adelgid in Pennsylvania” Entomology<br />
Circular No. 128. Regulatory Horticulture 15, no. 1 (1989): 9–10.<br />
Ragenovich, I. R., and R. G. Mitchell. “Balsam Woolly Adelgid.” Forest Insect and Disease<br />
Leafl et 118. U.S. Department of Agriculture. www.fs.fed.us/r6/nr/fi d/fi dls/fi dl-118.pdf<br />
(accessed on April 13, 2009).<br />
Sidebottom, J. “Balsam Woolly Adelgid CTN-020.” North Carolina State University<br />
Cooperative Extension. www.ces.ncsu.edu/fl etcher/programs/xmas/ctnotes/ctn020.html<br />
(accessed on April 13, 2009).<br />
———. “Scouting Frasier Fir in North Carolina (AG-573)—Balsam Woolly Adelgid.”<br />
ipm.ncsu.edu/Scouting_Fraser_Fir/contents.html (accessed on April 13, 2009).<br />
Botrytis Blight<br />
Douglas, S. M. Disease Problems in Connecticut <strong>Christmas</strong> <strong>Tree</strong> Plantations, 2004–2005.<br />
New Haven: Connecticut Agricultural Experiment Station, 2006. vvv.caes.state.ct.us/<br />
FactSheetFiles/PlantPathology/fspp024f.htm (accessed October 7, 2009).<br />
———. Diseases of <strong>Christmas</strong> <strong>Tree</strong> Seedlings and Transplant Beds. New Haven: Connecticut<br />
Agricultural Experiment Station, 2008. www.ct.gov/caes/lib/caes/documents/publications/<br />
fact_sheets/plant_pathology_and_ecology/diseases_of_christmas_tree_seedling_and_transplant_beds_06-30-08r.pdf<br />
(accessed October 7, 2009).<br />
Cooley Spruce Gall Adelgid (on Spruce and Douglas-Fir)<br />
“Forest Health Fact Sheet: Cooley Spruce Gall Adelgid.” Pennsylvania Department of<br />
Conservation and Natural Resources. www.dcnr.state.ps.us/Forestry/leafl ets/cooley.htm<br />
(accessed on December 9, 2008).<br />
Hoover, G. A. “Cooley Spruce Gall Adelgid.” University Park: The Pennsylvania State<br />
University, 2002. ento.psu.edu/extension/factsheets/cooleyspgall.htm (accessed on<br />
December 9, 2008).<br />
Shetlar, D. J. “Cooley Spruce Gall Adelgid on Colorado Blue Spruce.” Ohio State<br />
University Extension Fact Sheet. ohioline.osu.edu/hyg-fact/2000/2552.html<br />
(accessed on December 9, 2008).<br />
———. “Cooley Spruce Gall Adelgid on Douglas-fi r.” Ohio State University Extension<br />
Fact Sheet. ohioline.osu.edu/hyg-fact/2000/2551.html (accessed on December 9, 2008).<br />
Stimmel, J. F. “Cooley Spruce Gall Aphid, Adelges cooleyi.” Entomology Circular No. 51.<br />
Regulatory Horticulture 6, no. 2 (1980): 13–14.<br />
Cryptomeria Scale<br />
Gardosik, S. M. “Aspiditus cryptomeriae Kuwana, an Armored Scale <strong>Pest</strong> of Conifers.”<br />
Entomology Circular No. 200. Regulatory Horticulture 27 (2001): 23–26.<br />
IPM FOR CHRISTMAS TREE PRODUCTION ......................................................................................................................................................................................................................................... 188
Cyclaneusma Needle Cast<br />
Peterson, G. W., and J. A. Walla. “Naemacyclus (Cyclaneusma) Needle Cast of Pines.”<br />
In Diseases of <strong>Tree</strong>s in the Great Plains, coord. by Jerry W. Riffl e and Glenn W. Peterson,<br />
122–23. www.unl.edu/nac/diseasetrees (accessed on February 23, 2009).<br />
Sidebottom, J. “Needle Cast Diseases in <strong>Christmas</strong> <strong>Tree</strong>s CTN-033.” North Carolina State<br />
University Cooperative Extension. www.ces.ncsu.edu/fl etcher/programs/xmas/ctnotes/<br />
ctn033.html (accessed on February 23, 2009).<br />
Taylor, N. J., and S. T. Nameth. “Needle Diseases on 2-Needled Conifers in Ohio.”<br />
Ohio State University Extension Fact Sheet. ohioline.osu.edu/hyg-fact/3000/3071.html<br />
(accessed on February 23, 2009).<br />
Douglas-Fir Needle Midge<br />
Carleo, J. “The Douglas-fi r Needle Midge.” In Plant and <strong>Pest</strong> Advisory Landscape, Nursery<br />
and Turf Edition. Rutgers Cooperative Research and Extension at the New Jersey Agricultural<br />
Experiment Station. njaes.rutgers.edu/pubs/plantandpestadvisory/2005/ln0728.pdf<br />
(accessed on March 2, 2009).<br />
DeAngelis, J. D. “Biology and Control of Douglas-fi r Needle Midge in <strong>Christmas</strong> <strong>Tree</strong>s.”<br />
Oregon State University Extension Service. extension.oregonstate.edu/catalog/pdf/ec/<br />
ec1373-e.pdf (accessed on March 2, 2009).<br />
Diplodia (Sphaeropsis) Blight<br />
“Diplodia Tip Blight.” Cornell University Plant Disease Diagnostic Clinic. plantclinic<br />
.cornell.edu/factsheets/diplodia/diplodia.htm (accessed on May 28, 2009).<br />
Douglas, S. M. “Diplodia Tip Blight (Sphaeropsis Blight).” Connecticut Agricultural<br />
Experiment Station. vvv.caes.state.ct.us/FactSheetFiles/PlantPathology/fspp016f.htm<br />
(accessed on May 28, 2009).<br />
Gallenberg, D., and T. Chase. “Diplodia Shoot Blight of Pines.” South Dakota State<br />
University Cooperative Extension. agbiopubs.sdstate.edu/articles/ExEx8075.pdf<br />
(accessed on May 28, 2009).<br />
Gillman, D. H. “Sphaeropsis (Diplodia) Blight.” University of Massachusetts Extension.<br />
www.umassgreeninfo.org/fact_sheets/diseases/sphaeropsis_diplodia_blight.pdf<br />
(accessed on May 28, 2009).<br />
“GreenShare Factsheet: Diplodia Tip Blight of Pines.” University of Rhode Island<br />
Landscape Horticulture Program. www.uri.edu/ce/factsheets/sheets/diplodiapine.html<br />
(accessed on May 28, 2009).<br />
Peterson, G. W. “Diplodia Blight of Pines: Forest Insect and Disease Leafl et 16.” U.S.<br />
Department of Agriculture. www.na.fs.fed.us/SPFO/pubs/fi dls/diplodia/diplodiafi dl.htm<br />
(1981) (accessed on May 28, 2009)<br />
Eastern Pine Weevil<br />
Lehman, R. D. “Northern Pine Weevil, Pissodes approximatus Hopkins.” Entomology<br />
Circular No. 111. Regulatory Horticulture 13, no. 2 (1987).<br />
Eastern Spruce Gall Adelgid<br />
“Eastern Spruce Gall Adelgid.” University of Massachusetts Extension Fact Sheet. www<br />
.umassgreeninfo.org/fact_sheets/galls/eastern_spruce.html (accessed on January 9, 2009).<br />
BIBLIOGRAPHY .................................................................................................................................................................................................................................................................................... 189
Technical Sources (continued)<br />
“Forest Health Fact Sheet: Eastern Spruce Gall Adelgid.” Department of Conservation<br />
and Natural Resources. www.dcnr.state.ps.us/Forestry/leafl ets/eastspruce.htm<br />
(accessed on January 9, 2009).<br />
Hoover, G. A. “Eastern Spruce Gall Adelgid.” University Park: The Pennsylvania State<br />
University, 2001. ento.psu.edu/extension/factsheets/e_spruce_gall_adelgid.htm<br />
(accessed on January 9, 2009).<br />
Salsedo, C. A., and E. L. Marrotte. “Spruce Gall Adelgids.” University of Connecticut<br />
Cooperative Extension. www.hort.uconn.edu/IPM/homegrnd/htms/59spgall.htm<br />
(accessed on January 9, 2009).<br />
Shetlar, D. J. “Eastern Spruce Gall Adelgid.” Ohio State University Extension Fact Sheet.<br />
ohioline.osu.edu/hyg-fact/2000/2550.html (accessed on January 9, 2009).<br />
Elongate Hemlock Scale<br />
Hoover, Greg. “Elongate Hemlock Scale.” University Park: The Pennsylvania State<br />
University, 2003. ento.psu.edu/extension/factsheets/elongate-hemlock-scale<br />
(accessed on January 19, 2009).<br />
Keystone <strong>Tree</strong> Experts. “Elongate Hemlock Scale.” Keystone <strong>Tree</strong> Experts: Horticultural<br />
Consultants. www.keystonetree.com/insect_and_disease_index.htm?p=6592,80567<br />
(accessed on January 19, 2009).<br />
Malinoski, M. “Scale Insects on Hemlock.” Home and Garden Mimeo No. HG 81.<br />
Maryland Cooperative Extension. www.hgic.umd.edu/_media/documents/hg81.pdf<br />
(accessed on January 19, 2009).<br />
McClure, M. S. “<strong>Pest</strong> Alert—Elongate Hemlock Scale.” U.S. Department of Agriculture.<br />
www.na.fs.fed.us/spfo/pubs/pest_al/ehscale/ehscale.htm (accessed on January 19, 2009).<br />
Stimmel, J. F. “Elongate Hemlock Scale.” Entomology Circular No. 36. Regulatory<br />
Horticulture 5, no. 1 (1979): 13–14.<br />
Environmental Stresses<br />
Bäck, J., S. Huttunen, and M. Roitto. “Pollutant Response and Ecophysiology of Conifers<br />
in Open-Top Chamber Experiments.” Aquilo Ser. Botanica 32 (1993): 9–19.<br />
Bartholomay, G. A., R. T. Eckert, and K. T. Smith. “Reductions in <strong>Tree</strong>-Ring Widths of<br />
White Pine Following Ozone Exposure at Acadia National Park, Maine, U.S.A.”<br />
Canadian Journal of Forest Research 27 (1997): 361–68.<br />
MacLean, D. C., R. E. Schneider, and L. H. Weinstein. “Response of Five Species of<br />
Conifers to Sulfur Dioxide and Hydrogen Fluoride during Winter Dormancy.”<br />
Environmental and Experimental Botany 26 (1985): 291–96.<br />
Pellett, H., R. Hummel, and L. Mianquist. “Relationship of Fall Watering Practice to<br />
Winter Injury.” Journal of Arboriculture 6 (1980): 146–49.<br />
Swift, C. E., W. R. Jacobi, M. Schomaker, and D. A. Leatherman. Environmental Disorders<br />
of Woody Plants. Fort Collins: Colorado State University Extension, 2008.<br />
IPM FOR CHRISTMAS TREE PRODUCTION ......................................................................................................................................................................................................................................... 190
Eriophyid Mites<br />
Day, E. “Pine Bud Mite/Needle Sheath Mite.” Virginia Polytechnic Institute and State<br />
University Cooperative Extension. www.ext.vt.edu/departments/entomology/factsheets/<br />
pibunmit.html (accessed on January 13, 2009).<br />
Lehman, R. D. “Eriophyid Mites—An Introduction.” Entomology Circular No. 117.<br />
Regulatory Horticulture 13, no. 2 (1987): 23–25.<br />
Sidebottom, J. “Rust Mites on <strong>Christmas</strong> <strong>Tree</strong>s CTN-034.” North Carolina State University<br />
Cooperative Extension. www.ces.ncsu.edu/fl etcher/programs/xmas/ctnotes/ctn034.html<br />
(accessed on January 13, 2009).<br />
———. “Scouting Frasier Fir in North Carolina (AG-573)—Rust Mites.” ipm.ncsu.edu/<br />
Scouting_Fraser_Fir/contents.html (accessed on January 13, 2009).<br />
Gall Rusts<br />
“Eastern Gall Rust.” Insects and Diseases of <strong>Tree</strong>s in the South. USDA Forest Service Forest<br />
Health Protection, Region 8. fhpr8.srs.fs.fed.us/<strong>for</strong>stpst.html (accessed October 7, 2009).<br />
Merrill, W. “Pine-Oak Gall Rust.” <strong>Christmas</strong> <strong>Tree</strong> <strong>Pest</strong> Note No. 201. University Park:<br />
The Pennsylvania State University, 1987.<br />
Merrill, W., and N. Wenner. “Pine-Pine Gall Rust.” <strong>Christmas</strong> <strong>Tree</strong> <strong>Pest</strong> Note No. 200.<br />
University Park: The Pennsylvania State University, 1987.<br />
Growing Degree Day Ranges <strong>for</strong> <strong>Christmas</strong> <strong>Tree</strong> <strong>Pest</strong>s<br />
Adams, N. E. “Using Growing Degree Days <strong>for</strong> Insect <strong>Management</strong>.” University of New<br />
Hampshire Cooperative Extension. extension.unh.edu/Agric/GDDays/Docs/growch.pdf<br />
(assessed November 1, 2009).<br />
Clark, S., and T. Kowalsick. “Using Growing Degree-Days <strong>for</strong> Insect <strong>Pest</strong> <strong>Management</strong>.”<br />
Cornell University Cooperative Extension, Suffolk County. ccesuffold.org/assets/<br />
Horticulture-Leafl ets/Using-Growing-Degree-Days-<strong>for</strong>-Insect-<strong>Pest</strong>-<strong>Management</strong>.pdf<br />
(accessed April 4, 2008).<br />
Couch, G. <strong>Pest</strong> <strong>Management</strong> Guide <strong>for</strong> Commercial <strong>Production</strong> and Maintenance of <strong>Tree</strong>s<br />
and Shrubs. Ithaca: Cornell University Cooperative Extension, 2009. ipmguidelines.org/<br />
treesandshrubs/ (accessed November 1, 2009).<br />
Gardosik, S. “Pennsylvania <strong>Christmas</strong> <strong>Tree</strong> Scouting Reports.” Harrisburg: Bureau of Plant<br />
Industry, Pennsylvania Department of Agriculture, 2001–2007. ento.psu.edu/extension/<br />
christmas-trees/scouting-reports.<br />
“Landscape Degree Day In<strong>for</strong>mation.” Michigan State University, <strong>Integrated</strong> <strong>Pest</strong><br />
<strong>Management</strong> Resources. www.ipm.msu.edu/landscapeipm/gddlandchart.htm<br />
(accessed November 23, 2009).<br />
Orton, D. A., and T. L. Green. Coincide. Flossmoor, Ill.: Plantsmen’s Publications, 1989.<br />
Gypsy Moth<br />
“2009 Gypsy Moth Suppression Program.” Department of Conservation and Natural<br />
Resources. www.dcnr.state.ps.us/Forestry/gypsymoth/index.aspx (accessed on May 13, 2009).<br />
Hoover, G. “Elongate Hemlock Scale.” University Park: The Pennsylvania State University,<br />
2000. ento.psu.edu/extension/factsheets/gypsy-moth (accessed on May 13, 2009).<br />
BIBLIOGRAPHY .................................................................................................................................................................................................................................................................................... 191
Technical Sources (continued)<br />
McManus, M., N. Schneeberger, R. Reardon, and G. Mason. “Gypsy Moth: Forest Insect<br />
and Disease Leafl et 162.” U.S. Department of Agriculture. www.na.fs.fed.us/SPFO/pubs/<br />
fi dls/gypsymoth/gypsy.htm (accessed on May 13, 2009).<br />
IPM Basics<br />
Flint, M. L., and P. Gouveia. IPM in Practice: Principles and Methods of <strong>Integrated</strong> <strong>Pest</strong><br />
<strong>Management</strong>. Davis: University of Cali<strong>for</strong>nia Agriculture and Natural Resources, 2001.<br />
Hill, N. S. Biological Control of Insects with Insect-Pathogenic Nematodes—A Brief Status Report.<br />
Harrisburg: Bureau of Plant Industry, Pennsylvania Department of Agriculture, 1997.<br />
Lehman, R. D. Growing Degree Days. Harrisburg: Bureau of Plant Industry, Pennsylvania<br />
Department of Agriculture, 1997.<br />
———. “Insecticidal Soap as an Alternative.” Harrisburg: Bureau of Plant Industry,<br />
Pennsylvania Department of Agriculture, 1997.<br />
———. “Repeat Applications.” Harrisburg: Bureau of Plant Industry, Pennsylvania<br />
Department of Agriculture, 1997.<br />
———. “Thresholds.” Harrisburg: Bureau of Plant Industry, Pennsylvania Department of<br />
Agriculture, 1997.<br />
Lehman, R. D., and J. F. Stimmel. “Dormant Oils.” Harrisburg: Bureau of Plant Industry,<br />
Pennsylvania Department of Agriculture, 1997.<br />
———. “Spray Adjuvants.” Harrisburg: Bureau of Plant Industry, Pennsylvania Department<br />
of Agriculture, 1997.<br />
Stimmel, J. F. “Bt—Inside and Out.” Harrisburg: Bureau of Plant Industry, Pennsylvania<br />
Department of Agriculture, 1997.<br />
——— “Considering Natural Enemies.” Harrisburg: Bureau of Plant Industry, Pennsylvania<br />
Department of Agriculture, 1997.<br />
Thomas, C. Greenhouse IPM with an Emphasis on Biocontrols. University Park: The Pennsylvania<br />
State University, 2005.<br />
Valley, K. “Neem Seed Extracts as <strong>Pest</strong>icidal Agents.” Harrisburg: Bureau of Plant Industry,<br />
Pennsylvania Department of Agriculture, 1997.<br />
Japanese Beetle<br />
Stimmel, J. F. “Japanese Beetle, Popillia japonica Newman.” Entomology Circular No. 76.<br />
Regulatory Horticulture 8, no. 2 (1982).<br />
Lophodermium Needle Cast<br />
“Lophodermium Needle Cast.” Center <strong>for</strong> Invasive Species and Ecosystem Health<br />
Bugwood Network, University of Georgia, www.<strong>for</strong>estpests.org/nursery/lophodermium.html<br />
(accessed on March 4, 2009).<br />
“Lophodermium Needle Cast.” Department of Plant Pathology, Cornell University.<br />
www.plantpath.cornell.edu/trees/LophNcst.html (accessed on March 4, 2009).<br />
Pecknold, P. C. “Lophodermium Needle Cast.” Purdue University Cooperative Extension<br />
Service. www.extension.purdue.edu/extmedia/BP/BP-52.pdf (accessed on March 4, 2009).<br />
IPM FOR CHRISTMAS TREE PRODUCTION ......................................................................................................................................................................................................................................... 192
Mechanical Damage<br />
Brand, M. H., ed. Southern New England <strong>Christmas</strong> <strong>Tree</strong> Growers’ Manual. Storrs: University<br />
of Connecticut Cooperative Extension, 1992.<br />
Kammerbauer, H., H. Selinger, R. Römmelt, A. Ziegler Jöns, D. Knoppik, and B. Hock.<br />
“Toxic Effects of Exhaust Emissions on Spruce Picea abies and Their Reduction by the<br />
Catalytic Converter.” Environmental Pollution 42 (1986): 133–42.<br />
Pales Weevil<br />
Day, E., and S. Salom. “Pales Weevil.” Virginia Polytechnic Institute and State University<br />
Cooperative Extension. pubs.ext.vt.edu/2902/2902-1102/2902-1102.html (accessed on<br />
June 9, 2009).<br />
Nord, J. C., I. Ragenovich, and C. A. Doggett. “Pales Weevil.” Forest Insect and Disease<br />
Leafl et 104. U.S. Department of Agriculture. www.na.fs.fed.us/SPFO/pubs/fi dls/pales/<br />
fi dl-pales.htm (accessed on June 9, 2009).<br />
“Pales Weevil.” Maine Forest Service, Maine Department of Conservation. www.maine<br />
.gov/doc/mfs/paleswee.htm (accessed on June 9, 2009).<br />
Steinhauer, J. R. “Pales Weevil, Hylobius pales.” Entomology Circular No. 56. Regulatory<br />
Horticulture 7, no. 1 (1981): 17–18.<br />
<strong>Pest</strong>icide In<strong>for</strong>mation<br />
ESA Basics: More Than 30 Years of Conserving Endangered Species. Endangered Species Program,<br />
U.S. Fish and Wildlife Service. www.fws.gov/Endangered/factsheets/ESA_basics.pdf<br />
(accessed November 2, 2009).<br />
Phytophthora Root Rot<br />
Abbey, T. “Phytophthora Dieback and Root Rot.” <strong>Integrated</strong> <strong>Pest</strong> <strong>Management</strong>, University<br />
of Connecticut Cooperative Extension. www.hort.uconn.edu/Ipm/homegrnd/htms/42rhod<br />
.htm (accessed October 9, 2009).<br />
Merrill, W. “Phytophthora Root Rot.” <strong>Christmas</strong> <strong>Tree</strong> <strong>Pest</strong> Note No. 305. University Park:<br />
The Pennsylvania State University, 1989.<br />
Pine Bark Adelgid<br />
Mazzey, K., and M. Masiuk. “Pine Bark Adelgid.” Penn State Cooperative Extension.<br />
woodypests.cas.psu.edu/factsheets/InsectFactSheets/html/Pine_BarkA.html<br />
(accessed September 21, 2009).<br />
Shetlar, D. J. “Pine Bark Adelgid.” Bug Doc <strong>Christmas</strong> <strong>Tree</strong> Fact Sheet. Ohio State University.<br />
bugs.osu.edu/%7Ebugdoc/Shetlar/factsheet/christmasstree/images/PineBarkAdelgid/<br />
PinebarkAdlg.PDF (accessed September 21, 2009).<br />
Stimmel, J. F. “Pine Bark Adelgid, Pineus strobi (Hartig).” Entomology Circular No. 84.<br />
Regulatory Horticulture 10, no. 1 (1984).<br />
Pine Needle Scale<br />
Mazzey, K., and M. Masiuk. “Woody Ornamental IPM: Pine Needle Scale.” Penn State<br />
Cooperative Extension. woodypests.cas.psu.edu/FactSheets/InsectFactSheets/pdf/<br />
PineNeedleScale.pdf (accessed on December 3, 2008).<br />
Shetlar, D. J. “Pine Needle Scale.” Ohio State University Extension Fact Sheet.<br />
ohioline.osu.edu/hyg-fact/2000/2553.html (accessed on December 3, 2008).<br />
Stimmel, J. F. “Pine Needle Scale, Chionaspis pinifoliae.” Entomology Circular No. 32.<br />
Regulatory Horticulture 4, no. 2. (1978): 15–16.<br />
BIBLIOGRAPHY .................................................................................................................................................................................................................................................................................... 193
Technical Sources (continued)<br />
Pine Root Collar Weevil<br />
Henry, T. J., and L. W. Bennett. “Pine Root Collar Weevil, Hylobius radicis Buchanan.”<br />
Entomology Circular No. 47. Regulatory Horticulture 6, no. 1 (1980).<br />
Lehman, R. D. “Pine Root Collar Weevil, Hylobius radicis Buchanan, in Pennsylvania.”<br />
Entomology Circular No. 122. Regulatory Horticulture 14, no. 1 (1988).<br />
Pine Sawfl ies<br />
“European Pine Sawfl y.” Department of Plant Pathology, Cornell University.<br />
www.plantpath.cornell.edu/trees/EuroPsawf.html (accessed on August 28, 2009).<br />
“Forest Health Fact Sheet: Introduced Pine Sawfl y.” Department of Conservation and<br />
Natural Resources. www.dcnr.state.ps.us/Forestry/leafl ets/introsawfl y.htm (accessed on<br />
August 28, 2009).<br />
“Forest Health Fact Sheet: Redheaded Pine Sawfl y.” Department of Conservation and<br />
Natural Resources. www.dcnr.state.ps.us/Forestry/leafl ets/redsawfl y.htm (accessed on<br />
August 28, 2009).<br />
Mazzey, K., and M. Masiuk. “Woody Ornamental IPM: European Pine Sawfl y Fact Sheet.”<br />
Penn State Cooperative Extension. woodypests.cas.psu.edu/FactSheets/InsectFactSheets/<br />
html/European_Pine_Sawfl y.html (accessed on August 28, 2009).<br />
McCullough, D. G., and N. W. Siegert. “Identifi cation and <strong>Management</strong> of European Pine<br />
Sawfl y.” Michigan State University Extension Bulletin E-2694. web1.msue.msu.edu/aoe/<br />
xmas/e-2694.PDF (accessed on August 28, 2009).<br />
“Redheaded Pine Sawfl y.” Forest Health Protection, Southern Region. www.fs.fed.us/r8/<br />
<strong>for</strong>esthealth/idotis/insects/redhdpsf.html (accessed on August 28, 2009).<br />
Shetlar, D. J. “European Pin Sawfl y.” Ohio State University Extension Fact Sheet<br />
HYG-25555-95. ohioline.osu.edu/hyg-fact/2000/2555.html (accessed on August 28, 2009).<br />
———. “Redheaded Pine Sawfl y.” Ohio State University Extension Fact Sheet HYG<br />
2554-95. ohioline.osu.edu/hyg-fact/2000/2554.html (accessed on August 28, 2009).<br />
Stimmel, J. F. “Introduced Pine Sawfl y, Diprion similis.” Entomology Circular No. 55.<br />
Regulatory Horticulture 7, no. 1 (1981): 15–16.<br />
———. “Redheaded Pine Sawfl y, Neodiprion lecontei (Fitch), and European Pine Sawfl y,<br />
Neodiprion sertifer (Geoff.).” Entomology Circular No. 54. Regulatory Horticulture 7,<br />
no. 1 (1981): 13–14.<br />
Wilson, Louis F. “Introduced Pine Sawfl y: Forest Insect and Disease Leafl et 99.” U.S.<br />
Department of Agriculture. www.na.fs.fed.us/SPFO/pubs/fi dls/intro_sawfl y/intro_sawfl y.htm<br />
(accessed on August 28, 2009).<br />
Wilson, Louis F., and Robert D. Averill. “Redheaded Pine Sawfl y: Forest Insect and Disease<br />
Leafl et 14.” U.S. Department of Agriculture. na.fs.fed.us/spfo/pubs/fi dls/pine_sawfl y/<br />
pinesawfl y.htm (accessed on August 28, 2009).<br />
Pine Shoot Beetle<br />
“Common Pine Shoot Beetle.” Department of Plant Pathology, Cornell University.<br />
www.plantpath.cornell.edu/trees/CPSBeetle.html (accessed on July 21, 2009).<br />
IPM FOR CHRISTMAS TREE PRODUCTION ......................................................................................................................................................................................................................................... 194
Haack, B., and D. Kucera. “<strong>Pest</strong> Alert—New Introduction: Common Pine Shoot Beetle.”<br />
U.S. Department of Agriculture. www.na.fs.fed.us/spfo/pubs/pest_al/shootbeetle/<br />
shootbeetle.htm (accessed on July 21, 2009).<br />
Lehman, R. D. “Common Pine Shoot Beetle, Tomicus piniperda.” Entomology Circular<br />
No. 151. Regulatory Horticulture 18, no. 2 (1992): 31–33.<br />
Pinewood Nematode<br />
“Pinewood Nematode, caused by Bursaphelenchus xylophilus.” Forest Health Protection,<br />
Region 8, USDA Forest Service. www.fs.fed.us/r8/<strong>for</strong>esthealth/<strong>for</strong>estpests/diseases/<br />
pnwdnema.pdf (accessed October 2, 2009).<br />
“Pinewood Nematode: Identifi cation, Biology, and <strong>Management</strong>.” The F. A. Bartlett <strong>Tree</strong><br />
Expert Company. www.bartlett.com/resources/Pinewood-Nematode.pdf (accessed October<br />
2, 2009).<br />
Ploioderma Needle Cast<br />
Gillman, D. H. “Ploioderma Needle Cast.” University of Massachusetts Extension Fact<br />
Sheet. www.umassgreeninfo.org/fact_sheets/diseases/ploioderma_needle_cast.pdf<br />
(accessed on July 15, 2009).<br />
“Ploioderma Needle Cast.” Department of Plant Pathology, Cornell University.<br />
www.plantpath.cornell.edu/trees/PloioNcst.html (accessed on July 15, 2009).<br />
Red-band Needle Blight (Dothistroma)<br />
“Dothistroma Needle Blight.” University of Illinois Extension. urbanext.illinois.edu/<br />
ShrubSelector/detail_problem.cfm?PathogenID=58 (accessed on February 18, 2009).<br />
Peterson, G. W. “Dothistroma Needle Blight of Pines: Forest Insect and Disease Leafl et<br />
143.” U.S. Department of Agriculture. na.fs.fed.us/spfo/pubs/fi dls/dothistroma/doth.htm<br />
(1982) (accessed on February 18, 2009).<br />
Peterson, G. W., and D. S. Wysong. “Dothistroma Blight of Pines.” In Diseases of <strong>Tree</strong>s in the<br />
Great Plains, coord. by Jerry W. Riffl e and Glenn W. Peterson, 120–22. www.unl.edu/nac/<br />
diseasetrees (accessed on February 18, 2009).<br />
Wagner, V. R. “Dothiostroma Needle Blight of Pine.” Plant Pathology Circular No. 36.<br />
Regulatory Horticulture 9, no. 1–2 (1983): 9–10.<br />
Rhabdocline Needle Cast<br />
Douglas, S. M. “Needle Casts of Douglas-Fir.” Connecticut Agricultural Experiment<br />
Station. www.ct.gov/caes/lib/caes/documents/publications/fact_sheets/plant_pathology<br />
_and_ecology/needlecasts_of_douglas-fi r_04-15-08r.pdf (accessed on February 6, 2009).<br />
Pscheidt, J. W. “Fir, Douglas—Needle Cast (Rhabdocline).” Oregon State University.<br />
plant-disease.ippc.orst.edu/disease.cfm?RecordID=448 (accessed on February 12, 2009).<br />
“Rhabdocline Needle Cast.” Department of Plant Pathology, Cornell University.<br />
www.plantpath.cornell.edu/trees/RhabdoN.html (accessed on February 12, 2009).<br />
Skilling, D. D., and H. L. Morton. “How to Identify and Control Rhabdocline and Swiss<br />
Needle Casts of Douglas-Fir.” U.S. Department of Agriculture. www.na.fs.fed.us/spfo/pubs/<br />
howtos/ht_df-ndlcst/ndlcst.htm (accessed on February 6, 2009).<br />
Wagner, V. R. “Rhabdocline Needle Cast.” Plant Pathology Circular No. 24.<br />
Regulatory Horticulture 6, no. 2 (2002).<br />
BIBLIOGRAPHY .................................................................................................................................................................................................................................................................................... 195
Technical Sources (continued)<br />
Rhizosphaera Needle Cast<br />
Heimann, M. F., and G. R. Stanosz. “Colorado Blue Spruce and Other Conifers Disorder:<br />
Rhizosphaera Needle Cast.” University of Wisconsin Extension Fact Sheet A2640.<br />
learningstore.uwex.edu/Assets/pdfs/A2640.pdf (accessed on February 12, 2009).<br />
Skilling, D. D., and J. A. Walla. “Rhizosphaera Needle Cast of Spruce.” In Diseases of <strong>Tree</strong>s<br />
in the Great Plains, coord. by Jerry W. Riffl e and Glenn W. Peterson, 124–25. www.unl.edu/<br />
nac/diseasetrees (accessed on February 12, 2009).<br />
Taylor, N. J., and S. T. Nameth. “Rhizosphaera Needle Cast on Spruce.” Ohio State<br />
University Extension Fact Sheet. ohioline.osu.edu/hyg-fact/3000/3059.html<br />
(accessed on February 12, 2009).<br />
Spruce Needle Rust<br />
Douglas, S. M. “Spruce Needle Rusts in Connecticut.” Connecticut Agricultural<br />
Experiment Station. www.ct.gov/CAES/cwp/view.asp?a=2815&q=376842<br />
(accessed on January 27, 2009).<br />
Hennon, P. E. “Spruce Needle Rust.” Forest Service, U.S. Department of Agriculture.<br />
www.fs.fed.us/r10/spf/fhp/leafl ets/Sprneerus.htm (2001) (accessed on January 27, 2009).<br />
Miller, K. J. “A Spruce Needle Rust.” Plant Pathology Circular No. 69.<br />
Regulatory Horticulture 19, no. 2 (1993).<br />
Stanosz, G. R. “Weir’s Cushion Rust of Spruces.” University of Wisconsin Extension.<br />
wihort.uwex.edu/gardenfacts/XHT1119.pdf (accessed on January 27, 2009).<br />
Spruce Spider Mite<br />
Lehman, R. D. “Spruce Spider Mite, Oligonychus ununguis (Jacobi)—An <strong>Integrated</strong><br />
Approach to <strong>Management</strong>.” Entomology Circular No. 190. Regulatory Horticulture 24,<br />
no. 1 (1998): 23–26.<br />
Sidebottom, J. “Spruce Spider Mite on Fraser Fir.” North Carolina State University<br />
Cooperative Extension. www.ces.ncsu.edu/fl etcher/programs/xmas/ctnotes/ctn029.html<br />
(accessed on November 3, 2008).<br />
“Spruce Spider Mite.” Cornell Cooperative Extension, Suffolk County. ccesuffolk.org/assets/<br />
Horticulture-Leafl ets/Spruce-Spider-Mite.pdf (accessed on October, 20, 2008).<br />
“Spruce.” In Woody Ornamental Insect, Mite, and Disease <strong>Management</strong>, edited by G. Moorman<br />
and G. Hoover. University Park: The Pennsylvania State University, 2008. woodypestguide<br />
.cas.psu.edu/141.htm (accessed on October 20, 2008).<br />
Striped Pine Scale<br />
Cranshaw, W. S. “Scale Insects Affecting Conifers.” Insect Series: <strong>Tree</strong>s and Shrubs<br />
No. 5.514. Colorado State University Cooperative Extension, 2006.<br />
Vertebrate <strong>Pest</strong>s<br />
Barnes, T. G. “Needle Points.” New Jersey <strong>Christmas</strong> <strong>Tree</strong> Growers Association, 2007.<br />
Belant, J. L., S. K. Ickes, L. A. Tyson, and T. W. Seamans. “Comparison of Four Particulate<br />
Substances as Wildlife Feeding Repellents.” Crop Protection 16 (1997): 439–47.<br />
Fergus, C. Wildlife of Pennsylvania and the Northeast. Mechanicsburg, Pa.: Stackpole Books,<br />
2000.<br />
Merritt, J. F. Guide to the Mammals of Pennsylvania. Pittsburgh: University of Pittsburgh<br />
Press, 1987.<br />
IPM FOR CHRISTMAS TREE PRODUCTION ......................................................................................................................................................................................................................................... 196
Nolte, D. L. 1998. “Effi cacy of Selected Repellents to Deter Deer Browsing on Conifer<br />
Seedlings.” International Biodeterioration and Biodegradation 42 (1998): 101–7.<br />
Sullivan, T. P., and D. S. Sullivan. “Vole-Feeding Damage and Forest Plantation Protection:<br />
Large-Scale Application of Diversionary Food to Reduce Damage to Newly Planted <strong>Tree</strong>s.”<br />
Crop Protection 27 (2008): 775–84.<br />
VerCauteren, K .C., M. J. Lavelle, and S. Hygnstrom. “Fences and Deer-Damage<br />
<strong>Management</strong>: A Review of Designs and Effi cacy.” Wildlife Society Bulletin 34 (2006):<br />
191–200.<br />
White Pine Blister Rust<br />
Merrill, W. “White Pine Blister Rust.” <strong>Christmas</strong> <strong>Tree</strong> <strong>Pest</strong> Note No. 306. University Park:<br />
The Pennsylvania State University, 1987.<br />
Wood Borers and Bark Beetles<br />
Connor, M. D., and R. C. Wilkinson. “Ips Bark Beetles in the South.” Forest Insect and<br />
Disease Leafl et 129. USDA Forest Service, 1983.<br />
Henry, T. J., and L. L. Signarovitz. “A Bark Beetle as a Potential <strong>Pest</strong> of Ornamental<br />
White Pine.” Entomology Circular No. 42. Regulatory Horticulture 5, no. 2 (1979).<br />
Kegley, S. J., R. L. Livingston, and K. E. Gibson. “Pine Engraver, Ips pini (Say), in the<br />
Western United States.” Forest Insect and Disease Leafl et 122. USDA Forest Service, 1997.<br />
Zimmerman Pine Moth<br />
Cranshaw, W. S. “Zimmerman Pine Moth.” <strong>Tree</strong>s and Shrubs, Insect Series No. 5.591.<br />
Colorado State Extension, 1999.<br />
Lehman, R. D. “Zimmerman pine moth, Dioryctria zimmermani.” Entomology Circular<br />
No. 192. Regulatory Horticulture 24, no. 2 (1998).<br />
BIBLIOGRAPHY .................................................................................................................................................................................................................................................................................... 197
GLOSSARY
Glossary<br />
Adelgid: members of the family Phylloxeridae (Homoptera), including gall, pine, and<br />
woolly adelgids, that are closely related to aphids but only found feeding on conifers, some<br />
<strong>for</strong>ming galls<br />
Aecium (aecia, pl.): a cup-shaped fruiting body of the rust fungi that produces aeciospores<br />
Aeciospores: rust fungi spores produced within aecia by infection<br />
Bacterium (bacteria, pl.): a single-celled, microscopic, plantlike organism that lacks<br />
chlorophyll and reproduces by fi ssion<br />
Basidiospore: an infectious spore produced by some rust fungi<br />
Bloom (glaucous bloom): the blue color of conifers (e.g., Colorado blue spruce) that is<br />
removed when sprayed with horticultural spray oil or insecticidal soap<br />
Brood: individuals hatching from eggs laid by one mother around the same time<br />
(including maturation later in life)<br />
Callow adults: condition of an adult shortly after emerging from the pupal case where its<br />
cuticle is not fully hardened or mature in color<br />
Cambium: a layer of actively dividing cells lying between the xylem and phloem in the<br />
stems and roots of vascular plants<br />
Candle: the growing, terminal shoot of certain conifers, particularly pines<br />
Canker: a disease of the bark and cambium that causes the tissue to become sunken or swollen<br />
Chlorosis: yellowing of normally green foliage or tissue due to chlorophyll destruction or<br />
failure of chlorophyll functions; often a symptom of some mineral defi ciency, extremely<br />
reduced light, root or stem girdling, pests, or viral infection<br />
Chlorotic (in terms of growth and spots): spots or splotching of normally green foliage due to<br />
a problem with the chlorophyll; often caused by insect feeding or disease<br />
Chrysalis: the pupal stage of butterfl ies; also known as the resting time between the larval<br />
and nymphal stages of mites<br />
Conidia (conidium, sing.): an asexual fungus spore<br />
Conidiophore: a specialized hypha bearing one or more conidia<br />
Contact dermatitis: a skin rash caused by an allergic reaction to something coming in<br />
contact with the skin (e.g., irritating hairs of gypsy moth caterpillar)<br />
Cull: removal of plants suspected to be infected of a disease, thus reducing the possibility of<br />
a spread of infection to plants not already affected<br />
Cuticle: with insects, the noncellular, thin, waxy outer layer of the body wall (exoskeleton)<br />
made up of wax and chitin; with plants, it is a thin, continuously waterproofed, noncellular<br />
fi lm on the external surface that tends to prevent desiccation and repels external water<br />
Cutin: the waxy substance comprising the inner layer of the cuticle; waterproof mixture<br />
of waxes, fatty acids, soaps, higher alcohols, and resinous materials <strong>for</strong>ming the chief<br />
ingredient of the cuticle of many plants<br />
IPM FOR CHRISTMAS TREE PRODUCTION ......................................................................................................................................................................................................................................... 200
Desiccate (desiccation): a loss of internal moisture content necessary to maintain growth,<br />
rigidity (helps keep the plant up), or survival; also known as dehydration or drying<br />
Epidermis: with insects, the cellular layer of the body wall that secretes the cuticle;<br />
with plants, the outermost layer of cells occurring on all the plant parts<br />
Epithelial cell (epithelial cells, pl.): layer of cells lining the resin duct<br />
Excelsior-like strands: fi ne-curled wood shavings that result from insect feeding in the wood<br />
Filament: a slender, threadlike structure<br />
Fissure: a narrow, longitudinal opening (slit)<br />
Fruiting body: a fungal organ specializing in the production of spores (e.g., aecium,<br />
apothecia, pycnidium, tilia)<br />
Gallery (galleries, pl.): a tunnel or chamber made by larvae of wood-boring insects and<br />
composed of silk and fecal waste; usually made by bark beetles (often <strong>for</strong>ming characteristic<br />
galleries that can be used in identifi cation) and miners and shoot, timber, and wood borers<br />
Host-specifi c: the degree to which an organism is limited to a specifi c type of host<br />
Hypha (hyphae, pl.): one of the fi laments of fungal mycelium<br />
Inoculum: a disease-causing propagule that can cause infection when brought into contact<br />
with the host<br />
Insect growth regulator (IGR): a pesticide built to imitate insect hormones that control<br />
molting and the development of some insect systems, affecting the change from immature<br />
to adult; in most cases, prevents the insect from becoming a sexually mature adult and may<br />
result in death<br />
Lesion: a localized area of discolored, diseased tissue resulting from an injury or wound<br />
Mottle (mottling): a spot or blotch of indistinct light and dark areas usually found on<br />
the needles<br />
Mycelia (mycelium, sing.): the threadlike, vegetative part of fungi; a mass of hyphae<br />
Necrotic: discolored or dead<br />
Niche (niches, pl.): a recessed compartment within a gallery where a female lays an egg<br />
Nuptial chamber: a large chamber created by male bark beetles used <strong>for</strong> mating with<br />
multiple females<br />
Oviposition: laying or depositing of eggs<br />
Ovipositor: the female insect’s egg-laying apparatus (external genitalia)<br />
Parasitoids: sometimes referred to as parasites and predators, these organisms feed in or on<br />
another living organism (host) over a long time span, consuming most to all of its tissues<br />
and resulting in death of the organism; behavior is intermediate between parasitism and<br />
predation<br />
Parthenogenesis (parthenogenetically): development of an egg without being fertilized<br />
GLOSSARY ........................................................................................................................................................................................................................................................................................... 201
Glossary (continued)<br />
Pathogen: a parasitic organism completely capable of causing disease<br />
Phloem: the layer of cells just inside bark of plants that conduct food from leaves to the<br />
stem and roots; different from xylem by the absence of thickened cells and the presence<br />
of cells containing sievelike areas<br />
Proleg (prolegs, pl.): a fl eshy, abdominal leg of certain insect larvae<br />
Pupate: larva trans<strong>for</strong>ming into a pupa<br />
Pycnidia (pycnidium, sing.): specialized, spherical, fungal fruiting bodies of rust fungi<br />
that produce infectious spores<br />
Resin duct: a tube or duct lined with epithelial cells in a woody stem or leaf, especially<br />
in conifers, that secretes resin (e.g., sap)<br />
Rhizome (rhizomes, pl.): a horizontal stem of a plant typically located underground that<br />
sends out roots and shoots<br />
Rhizomorph (rhizomorphs, pl.): rootlike structure of a fungus consisting of an aggregation<br />
of parallel hyphae<br />
Rosette: cluster of leaves in crowded circles or spirals rising up from the stem base<br />
(e.g., dandelion)<br />
Sclerotia (sclerotium, sing.): fi rm, often rounded masses of hyphae that serve as resting bodies<br />
and are resistant to unfavorable conditions<br />
Senescence: life phase of part of or a whole plant that involves decreased ability to repair<br />
damage, decline, or other factors that eventually lead to natural death<br />
Sessile: attached or fastened; incapable of moving from place to place<br />
Spore: single- or multiple-celled reproductive unit of fungi; each is capable of germinating<br />
and reproducing the organism (fungi)<br />
Sporulation: to produce or release spores<br />
Stem mother: a female aphid that produces live, identical aphids just be<strong>for</strong>e or as buds break<br />
Stippled: small dead spots on the leaves caused by mites’ and insects’ piercing-sucking<br />
mouthparts—specifi cally, a loss of sap and the toxic effect of saliva on the leaf tissue<br />
Stomata: pores in plant leaves that control gas exchange (carbon dioxide and oxygen) and<br />
transpiration of a plant<br />
Xylem: plant tissue (of trees and woody shrubs) consisting of various cell types that transport<br />
water and dissolved substances to the leaves; the main water-conducting tissue and chief<br />
supporting system composed of wood cells responsible <strong>for</strong> conduction, food transport,<br />
storage, and support<br />
IPM FOR CHRISTMAS TREE PRODUCTION ......................................................................................................................................................................................................................................... 202
Notes
Notes
Inches<br />
0 1 2 3 4 5 6 7<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
7<br />
8<br />
9<br />
10<br />
Centimeters<br />
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 16 17 18 19<br />
TAPPING SHEET FOR SCOUTING<br />
24<br />
23<br />
22<br />
21<br />
20<br />
19<br />
18<br />
17<br />
16<br />
15<br />
14<br />
13<br />
12<br />
11<br />
10<br />
9<br />
8<br />
7<br />
6<br />
5<br />
4<br />
3<br />
2<br />
1
The PA IPM Program<br />
is a collaboration between the<br />
Pennsylvania Department of Agriculture and<br />
The Pennsylvania State University<br />
aimed at promoting<br />
<strong>Integrated</strong> <strong>Pest</strong> <strong>Management</strong><br />
in both agricultural and nonagricultural settings.<br />
College of<br />
Agricultural Sciences<br />
Penn State College of Agricultural Sciences research and cooperative extension programs are<br />
funded in part by Pennsylvania counties, the Commonwealth of Pennsylvania, and the U.S.<br />
Department of Agriculture.<br />
Visit Penn State’s College of Agricultural Sciences on the Web: agsci.psu.edu<br />
This publication is available from the Publications Distribution Center, The Pennsylvania State<br />
University, 112 Agricultural Administration Building, University Park, PA 16802. For in<strong>for</strong>mation<br />
telephone 814-865-6713.<br />
Where trade names appear, no discrimination is intended, and no endorsement by the Penn State<br />
Cooperative Extension or the College of Agricultural Sciences is implied.<br />
This publication is available in alternative media on request.<br />
The Pennsylvania State University is committed to the policy that all persons shall have equal access<br />
to programs, facilities, admission, and employment without regard to personal characteristics not<br />
related to ability, per<strong>for</strong>mance, or qualifi cations as determined by University policy or by state or<br />
federal authorities. It is the policy of the University to maintain an academic and work environment<br />
free of discrimination, including harassment. The Pennsylvania State University prohibits discrimination<br />
and harassment against any person because of age, ancestry, color, disability or handicap, national<br />
origin, race, religious creed, sex, sexual orientation, gender identity, or veteran status. Discrimination<br />
or harassment against faculty, staff, or students will not be tolerated at The Pennsylvania State<br />
University. Direct all inquiries regarding the nondiscrimination policy to the Affi rmative Action<br />
Director, The Pennsylvania State University, 328 Boucke Building, University Park, PA 16802-5901;<br />
Tel 814-865-4700/V, 814-863-1150/TTY.<br />
Produced by Ag Communications and Marketing<br />
© The Pennsylvania State University 2011<br />
Code # AGRS-117 2M11/10payne5060<br />
INTEGRATED<br />
PEST<br />
MANAGEMENT<br />
<strong>for</strong><br />
CHRISTMAS<br />
TREE<br />
PRODUCTION<br />
A GUIDE FOR<br />
PENNSYLVANIA<br />
GROWERS